- Department of Neurosurgery, Maastricht University Medical Center +, Maastricht, The Netherlands
- Department of Neurology, Maastricht University Medical Center +, Maastricht, The Netherlands
- Department of Neuroscience, Maastricht University Medical Center +, Maastricht, The Netherlands
- Department of Neurosurgery, Ondokuz Mayis University Hospital, Atakum-Samsun 55139, Samsun, Turkey
- Department of Neurosurgery, Radboud University Medical Center, Geert Grooteplein Zuid 10, 6525 GA Nijmegen, The Netherlands
Correspondence Address:
Felix S. Gubler
Department of Neurosurgery, Maastricht University Medical Center +, Maastricht, The Netherlands
Department of Neurosurgery, Radboud University Medical Center, Geert Grooteplein Zuid 10, 6525 GA Nijmegen, The Netherlands
DOI:10.4103/sni.sni_172_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Felix S. Gubler, Linda Ackermans, Pieter L. Kubben, Aysun Damci, Mark L. Kuijf, Mayke Oosterloo, R. Jeroen Vermeulen, Sarah Hescham, Ersoy Kocabicak, Erkan Kurt, Yasin Temel. Infections in deep brain stimulation: Shaving versus not shaving. 10-Oct-2017;8:249
How to cite this URL: Felix S. Gubler, Linda Ackermans, Pieter L. Kubben, Aysun Damci, Mark L. Kuijf, Mayke Oosterloo, R. Jeroen Vermeulen, Sarah Hescham, Ersoy Kocabicak, Erkan Kurt, Yasin Temel. Infections in deep brain stimulation: Shaving versus not shaving. 10-Oct-2017;8:249. Available from: http://surgicalneurologyint.com/surgicalint-articles/infections-in-deep-brain-stimulation-shaving-versus-not-shaving/
Abstract
Background:To report our experience of infections in deep brain stimulation (DBS) surgeries comparing shaving versus no shaving of cranial hair. Nonshaving is strongly preferred by patients due to aesthetic and psychological factors.
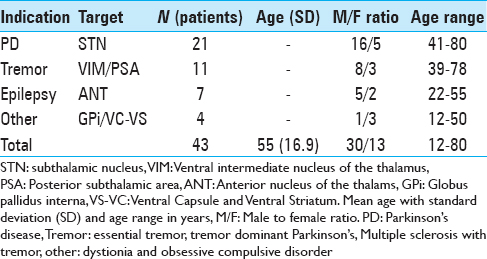
Methods:This study is a prospective follow-up of the infection rate in 43 nonshaven DBS cases between April 2014 and December 2015 compared to our former infection rate with shaving in our center. Minimum follow-up was 6 months. All patients, except 7 epilepsy patients, received implantation of the electrodes together with the extension cables and internal pulse generator in one session.
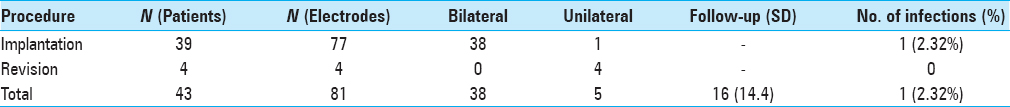
Results:In 43 nonshaven patients, a total of 81 electrodes were implanted or revised with a mean follow-up of 16 months. One patient (2.32%) developed an infection of the implanted DBS-hardware and was treated with antibiotics.
Conclusion:In our experience nonshaving of cranial hair in DBS surgery does not lead to more infections when compared to shaving. We have changed our protocol to nonshaving based on these findings.
Keywords: Complication, deep brain stimulation, infection, shaving
INTRODUCTION
Shaving of hair in cranial neurosurgery is still a common practice to reduce the risk of infections. However, there is more and more evidence that shaving does not decrease the risk of wound infection.[
Our philosophy in DBS surgeries was to entirely shave the hair of the patient to minimize the risk of infection. This has been the case from 1999 until 2014. However, with increasing application of DBS in younger patients with dystonia, epilepsy, and early stage Parkinson's disease (PD), patients indicated a strong preference towards nonshaving if possible. The latest dataset on shaving or not shaving in DBS was published 15 year ago and suggested that unshaved patients had no increased infection risk.[
In this study, we have prospectively investigated the infection rate of DBS surgeries in unshaved patients compared to our historical data of the infection rate of shaved patients.
MATERIALS AND METHODS
Study design
This study is a prospective observational study for the occurrence of postoperative infection of the DBS hardware. All patients receiving DBS at our center were monitored from the moment we stopped shaving in April 2014.
Data collection and follow-up
Between April 2014 and December 2015 all patients undergoing DBS were followed from the moment of operation. Indications included movement disorders, psychiatric disorders and epilepsy. Patient data including age, sex, diagnosis, DBS indications, and targets were reviewed [
Perioperative procedure
All patients undergoing DBS received the same treatment with the exception of shaving cranial hair. The evening before the operation hair was washed with povidon-iodine shampoo. On the morning of the surgery, before the stereotactic frame (Leksell, Elekta, Stockholm, Sweden) was mounted with local anesthesia, the head and hair of the patient was disinfected with colorless chlorhexidine solution (chlorhexidine digluconate 0.5% in alcohol 70%). Then, stereotactic computed tomography (CT) scan was performed to be fused with the previously performed magnetic resonance imaging (MRI), on which the anatomical planning was already done (Framelink, Medtronic, Minneapolis, USA). After positioning the patient on the operation table, again the head, hair, and frame were disinfected with chlorhexidine solution. Shortly after the chlorhexidine was dried, we placed both side pieces on the frame and the head was covered in a sterile manner with sterile surgical drapes and a large adhesive sterile transparent drape (Molnlycke Healthcare, Goteborg, Sweden). Small holes were cut in the transparent drape on both sides of the frame to expose the side pieces for mounting the arc on the frame. Both sides were sealed tightly with a povidon-iodine soaked gauze [
After the leads were placed, the distal ends were positioned in a subcutaneous pocket with a silicon protection (Medtronic, Minneapolis, USA). The wound was closed with a subcutaneous Vicryl suture and the skin was closed with one continuous Ethilon suture.
All patients underwent internalization in the same surgical session except for 7 epilepsy patients. These patients received implantation 5 days later due to electrophysiological recordings from the externalized leads.
Before implantation of the rest of the hardware, we removed the stereotactic frame and repositioned the patient. After marking the incisions on the cranial, infraclavicular, and abdominal site, we disinfected with povidon-iodine and covered with sterile surgical drapes to perform the rest of the operation with re-opening the cranial incision to connect the leads to the rest of the hardware. All incisions were closed with subcutaneous Vicryl sutures. Head incisions were closed with transcutaneous Ethilon sutures. The clavicular and abdominal incisions were closed with intracutaneous Monocryl sutures. PD and tremor patients recieved the electrode implantation awake with local anesthesia and were put under general anesthesia for the internalization part of the operation. Epilepsy, dystonia/dyskinetic cerebral palsy, and obsessive-compulsive disorder patients were under general anesthesia during the entire operation.
(Historical) analysis
The results of the postoperative wound infections without shaving related to the DBS hardware were prospectively followed-up and retrospectively analysed at our center. In addition, we compared the results to our historical infection rates with shaving.[
RESULTS
Infections
We observed one (2.32%) postoperative infection and compared it to our historical infection rate [
The operation on 11 February 2015 proceeded without complications, and 1 day later DBS was activated. On the fourth postoperative day, the patient developed a cough as well as fever (38.3°C), elevated infection parameters in the blood: C-reactive protein 41, white blood cell count 36, and sedimentation 12. Wound inspection and urine screening showed no abnormalities. Despite a negative chest X-ray, antibiotic therapy (ciprofloxacin orally twice daily 2750 mg) was started on suspicion of a hospital-acquired pneumonia, most likely based on patient's medical history. On the following days, infection parameters and fever worsened with dehiscence and puss from the retroauricular wound and a hematoma around the iPG. The wound was revised and the hematoma aspirated. Wound culture showed Staphylococcus aureus growth, for which flucloxacillin (6 times daily 1000 mg i.v.) was added. The infection subsided and infection parameters normalized. We decided on a multidisciplinary approach to treat the patient with ciprofloxacin and rifampicin because explanting the hardware was not an option in this case due to the severity of symptoms.[
Three days later, the patient was re-admitted to a local hospital with fever. Infection screening in blood, urine, sputum, cerebrospinal fluid, and gastrointestinal tract showed no abnormalities. Inspection of the iPG abdominal implantation site, retroauricular, and clavicular scars showed no signs of infection at that time. Nevertheless, the abdominal scar started to leak later for which the patient was admitted to our hospital on 14th April 2015. Sadly, the next morning he was found dead in his bed due to autoasphyxiation. No autopsy was performed on the request of the family.
DISCUSSION
Our results show that not shaving does not lead to more infections in DBS surgeries but might even lead to a lower risk. These results are in accordance with the current literature concerning cranial neurosurgery.[
In addition, a lower infection rate of 1.6% versus 0.5%, when not shaving, was found in a retrospective analysis concerning DBS surgeries.[
Our theory concerning the explanation of increased infections due to the shaving of hair comes down on a two-sided mechanism: the mechanical disruption of the skin's natural barrier and the disruption of the skin on a microenvironmental level. The introduction of traumatic skin lesions due to shaving leads to disruption of the skin's mechanical barrier. These shaving-induced lesions, visible or not, make the introduction of bacteria in the wound area more likely.[
This time interval related risk infection supports the mechanical skin barrier disruption theory. The longer before a surgery a patient is shaved, and thus the likelihood of introducing bacteria, the more time these bacteria will have to grow and proliferate to manifest in a possible wound infection.
On the other hand, we also think that shaving alters the skin's normal microenvironment which contains several microbiota including bacteria (Actinobacteria and Staphylococcaceae) and phyla. The skin flora in the normal patient is nonpathogenic, mostly commensal and in some cases mutualistic. Beneficial effects of skin bacteria are the prevention of colonizing of other transient pathogenic microorganism by competing for nutrients, secreting chemicals against them, or simulating the skin's immune response.[
A final note is that nonshaving is clearly preferred by neurosurgical patients due to psychological and aesthetic factors.[
Limitations
Limitations of this study are the small sample size and old historical compare group. Clinical significant equivalence or non-inferiority of not shaving cannot be shown by this small cohort of patients as reflected in the confidence interval and P value. The current historical comparison is the best available in our center.
CONCLUSION
In our experience not shaving in DBS surgery does not lead to more infections when compared to shaving of cranial hair. Not shaving is also highly preferred by patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Allegranzi B, Zayed B, Bischoff P, Kubilay NZ, de Jonge S, de Vries F. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect Dis. 2016. 16: e288-e303
2. Bekar A, Korfali E, Doğan S, Yilmazlar S, Başkan Z, Aksoy K. The effect of hair on infection after cranial surgery. Acta Neurochir (Wien). 2001. 143: 533-6
3. Broekman ML, van Beijnum J, Peul WC, Regli L. Neurosurgery and shaving: What's the evidence?. J Neurosurg. 2011. 115: 670-8
4. Cogen AL, Nizet V, Gallo RL. Skin microbiota: A source of disease or defence?. Br J Dermatol. 2008. 158: 442-55
5. Frati A, Pichierri A, Esposito V, Frati R, Delfini R, Cantore G. Aesthetic issues in neurosurgery: A protocol to improve cosmetic outcome in cranial surgery. Neurosurg Rev. 2007. 30: 69-76
6. Fukaya C, Yamamoto T. [Systematic review of complications for proper informed consent (13) stereotactic and functional neurosurgery]. No Shinkei Geka. 2014. 42: 751-68
7. Horgan MA, Piatt JH. Shaving of the scalp may increase the rate of infection in CSF shunt surgery. Pediatr Neurosurg. 1997. 26: 180-4
8. Kocabicak E, Temel Y. Deep brain stimulation of the subthalamic nucleus in Parkinson's disease: Surgical technique, tips, tricks and complications. Clin Neurol Neurosurg. 2013. 115: 2318-23
9. Kose G, Tastan S, Kutlay M, Bedir O. The effects of different types of hair shaving on the body image and surgical site infection in elective cranial surgery. J Clin Nurs. 2016. 25: 1876-85
10. Mangram AJ. A brief overview of the 1999 CDC Guideline for the Prevention of Surgical Site Infection. Centers for Disease Control and Prevention. J Chemother. 2001. 13: 35-9
11. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999. 20: 250-78
12. Miyagi Y, Shima F, Ishido K. Implantation of deep brain stimulation electrodes in unshaved patients. Technical note. J Neurosurg. 2002. 97: 1476-8
13. Ratanalert S, Saehaeng S, Sripairojkul B, Liewchanpattana K, Phuenpathom N. Nonshaved cranial neurosurgery. Surg Neurol. 1999. 51: 458-63
14. Sebastian S. Does preoperative scalp shaving result in fewer postoperative wound infections when compared with no scalp shaving? A systematic review. J Neurosci Nurs. 2012. 44: 149-56
15. Seropian R, Reynolds BM. Wound infections after preoperative depilatory versus razor preparation. Am J Surg. 1971. 121: 251-4
16. Siddique MS, Matai V, Sutcliffe JC. The preoperative skin shave in neurosurgery: Is it justified?. Br J Neurosurg. 1998. 12: 131-5
17. Smeets AY, Ackermans L, Oosterloo M, Kuijf ML, van Overbeeke JJ, Visser-Vandewalle V. Modified cement-based fixation of the deep brain stimulation electrode. Stereotact Funct Neurosurg. 2015. 93: 67-
18. Tang K, Yeh JS, Sgouros S. The Influence of hair shave on the infection rate in neurosurgery. A prospective study. Pediatr Neurosurg. 2001. 35: 13-7
19. Temel Y, Ackermans L, Celik H, Spincemaille GH, van der Linden C, Walenkamp GH. Management of hardware infections following deep brain stimulation. Acta Neurochir (Wien). 2004. 146: 355-61
20. Winston KR. Hair and neurosurgery. Neurosurgery. 1992. 31: 320-9