- Department of Neurosurgery, Hospital Italiano de Buenos Aires, Argentina
- Department of Neurology, Hospital Italiano de Buenos Aires, Argentina
Correspondence Address:
Federico Landriel
Department of Neurosurgery, Hospital Italiano de Buenos Aires, Argentina
DOI:10.4103/sni.sni_385_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Federico Landriel, Matteo Baccanelli, Santiago Hem, Eduardo Vecchi, Mariana Bendersky, Claudio Yampolsky. Intraoperative monitoring for spinal radiculomedullary artery aneurysm occlusion treatment: What, when, and how long?. 06-Sep-2017;8:211
How to cite this URL: Federico Landriel, Matteo Baccanelli, Santiago Hem, Eduardo Vecchi, Mariana Bendersky, Claudio Yampolsky. Intraoperative monitoring for spinal radiculomedullary artery aneurysm occlusion treatment: What, when, and how long?. 06-Sep-2017;8:211. Available from: http://surgicalneurologyint.com/?post_type=surgicalint_articles&p=8604
Abstract
Background:Spinal radiculomedullary artery aneurysms are extremely rare. Treatment should be tailored to clinical presentation, distal aneurysm flow, and lesion anatomical features. When a surgical occlusion is planned, it is necessary to evaluate whether intraoperative monitoring (IOM) should be considered as an indispensable tool to prevent potential spinal cord ischemia.
Methods:We present a patient with symptoms and signs of spinal subarachnoid hemorrhage resulting from the rupture of a T4 anterior radiculomedullary aneurysm who underwent open surgical treatment under motor evoked potential (MEP) monitoring.
Results:Due to the aneurysmal fusiform shape and preserved distal flow, the afferent left anterior radiculomedullary artery was temporarily clipped; 2 minutes after the clamping, the threshold stimulation level rose higher than 100 V, and at minute 3, MEPs amplitude became attenuated over 50%. This was considered as a warning criteria to leave the vessel occlusion. The radiculomedullary aneurysm walls were reinforced and wrapped with muscle and fibrin glue to prevent re-bleeding. The patient awoke from general anesthesia without focal neurologic deficit and made an uneventful recovery with complete resolution of her symptoms and signs.
Conclusion:This paper attempts to build awareness of the possibility to cause or worsen a neurological deficit if a radiculomedullary aneurysm with preserved distal flow is clipped or embolized without an optimal IOM control. We report in detail MEP monitoring during the occlusion of a unilateral T4 segmental artery that supplies an anterior radiculomedullary artery aneurysm.
Keywords: Spinal aneurysm, spinal cord ischemia, spinal subarachnoid hemorrhage, transcranial motor evoked potentials
BACKGROUND
Spinal radiculomedullary artery aneurysms are extremely rare. Djindjian et al.[
When a surgical occlusion is planned, we need to evaluate whether intraoperative monitoring (IOM) should be considered as an indispensable tool to prevent potential spinal cord ischemia. Several IOM techniques have been suggested to detect early spinal cord ischemia, such as somatosensory-evoked potentials (SSEP), transcranial motor evoked potentials (TcMEP), or epidural potentials (eMEP), a modification of TcMEP.[
MATERIALS AND METHODS
Case description
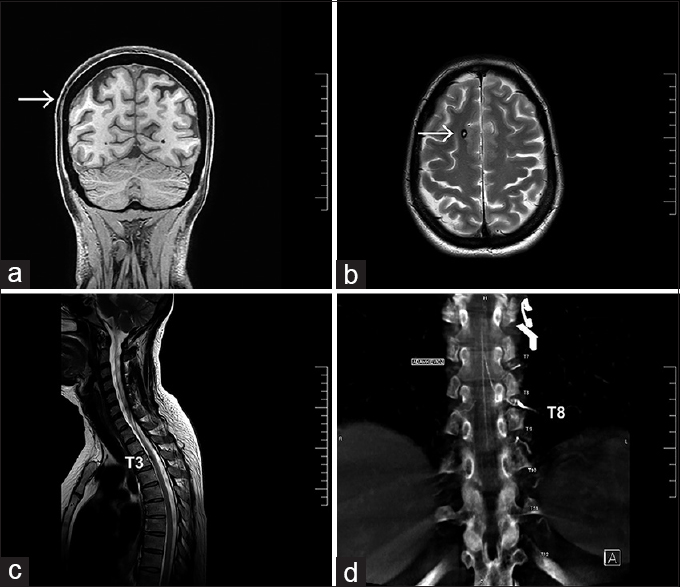
A 53-year-old previously healthy female was admitted with a 10-day history of acute thoracolumbar back pain with subsequent development of intense headache, nuchalgia, and mild meningeal irritation symptoms. She had a normal physical examination; Kerning, Brudzinski, and Lhermitte maneuvers were negative. Cranial computed tomography (CT) showed a subarachnoid hemorrhage on the right parietal convexity and a right frontal lesion compatible with a cavernoma [
Figure 1
(a) Coronal MRI view shows subarachnoid hemorrhage on the right parietal convexity (white arrow) (b) Axial MRI view demonstrates a right frontal lesion compatible with a Cavernoma (white arrow). (c) Sagittal MRI shows a small nodular formation at T3 spinal level. (d) Angio-tomography demonstrates Adamkiewicz artery arising from the T8 left segmental artery
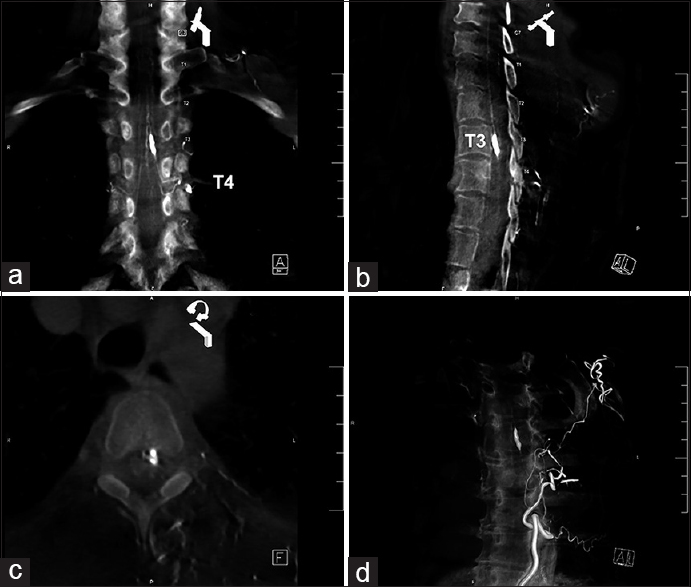
Figure 2
Angio-tomography 3D reconstruction. (a) Coronal view shows left T4 radiculomedullary aneurysm. (b) Antero-medullary location of the aneurysm al T3 spinal level. (c) Axial view shows left anterolateral location of the lesion. (d) Angio-CT 3D reconstruction of the fusiform anterior radiculomedullary artery aneurysm
Transcranial motor evoked potentials monitoring (threshold-level method)
The anesthetic protocol used for IOM monitoring always includes total intravenous anesthesia (TIVA) with Remifentanyl and Propofol and the use of muscular relaxants for intubation.
Corckscrew electrodes were placed just anterior to C3 and C4 (as defined by the international 10–20 system), stimulating the precentral gyrus. The stimulus consisted of a 4-pulse train with a 2 ms interpulse interval. Initial stimulus intensity was set at 100 V and increased at fixed increments of 50 V, limiting the maximum voltage to 500 V and reversing the electrode polarity between stimuli. Myogenic responses were recorded bilaterally with needle electrodes placed in the abductor digiti primi, tibialis anterior, and abductor hallucis muscles. A significant change in MEP threshold after clamping the segmental arteries was defined as a threshold increase of 100 V or more. The threshold method is more sensitive to corticospinal deterioration than the “all or none” method: it assumes that, under unchanged stimulation and anesthesia conditions, the stimulus voltage needed to elicit a minimal compound muscle action potential from a given target muscle will remain relatively constant. Deterioration in central motor conduction and/or lower motor neuron function will be reflected by a need for stronger stimulation intensity, recruiting a large population of upper motor neurons. Hence, if a larger stimulus is needed during segmental cross-clamping, it may reflect critical ischemia of the motor pathways.[
RESULTS
Surgical treatment (See
A bilateral laminectomy was performed at T3 and T4 levels. The dura was opened under microscope magnification and retracted to improve the surgical field. The anterior radiculomedullary left branch of the T4 segmental artery, the left nerve root, and the denticulate ligament were identified. The dental ligament was sectioned to improve lesion exposure. The spinal cord was gently retracted to the right. A fusiform aneurysm with strong adhesions to the anterolateral surface of the spinal cord and anterior dura was identified. The aneurysm was gently dissected from the yellowish anterior thickened dura. Afferent and distal arteries were identified with evidence of blood flow in the efferent vessel. Due to the aneurysmal fusiform shape and the preserved distal flow, the afferent left anterior radiculomedullary artery was temporary clipped; 2 minutes after the clamping, the threshold stimulation level raised over 100 V, at minute 3, MEPs amplitude became attenuated over 50%. Based on the rapid changes in the MEP, attempts to clip the aneurysm were abandoned. Temporary vessel occlusion was re-opened and MEP threshold and amplitude gradually returned to pre-clamping measures. The radiculomedullary aneurysm walls were reinforced and wrapped with muscle and fibrin glue to prevent re-bleeding. The patient awoke from general anesthesia without focal neurologic deficit and made an uneventful recovery with complete resolution of her symptoms and signs.
DISCUSSION
The goal of intraoperative electrophysiological monitoring (IOM) is to identify spinal cord ischemia that occurs during the procedure and to guide the intraoperative management to reduce the risks of neurological damage. The choice of the appropriate IOM technique requires understanding of spinal cord blood flow and of the spinal cord physiology, surgical technique, and their interaction.
Arterial supply to the spinal cord derives from the anterior spinal artery (ASA), posterior spinal artery (PSA), and segmental arteries. Perimedullary anastomoses between these arteries are numerous. In the thoracic spine, the segmental arteries originate from the aorta, and after coursing the lateral surface of the vertebral body, they divide on each side into three major branches: ventral (posterior intercostal), dorsal (muscular and cutaneous branches), and medial or spinal.[
The ligation of the segmental vessels is routinely performed during anterior spine instrumentation, although large series and reviews support the safety of this surgical maneuver,[
We have to consider three principal anatomical features: (1) only a few segmental arteries supply the spinal cord, (2) the anterior radiculomedullary arteries are 6 (range 2–14), whereas the posterior ones range from 11 to 16,[
Several authors have described different IOM methods to measure the potential risk of spinal ischemia by occluding the unilateral thoracic segmental artery: SSEP, TcMEP, or eMEP. However, the time limit to consider the sacrifice of a segmental artery safe in the absence of IOM alteration is poorly reported in the literature. Wu et al.[
Epidural MEPs are derived from TcMEPs and require the placement of a special epidural stimulator. TcMEPs are the most suitable technique to detect early spinal ischemia given that they measure corticospinal tract and lower motor neurons, supplied by the anterior spinal artery. The threshold-level described by Calancie et al. proved to be more sensitive to early corticospinal tract deterioration than the “all or none” method. Usually, when MEP threshold increases more than 100 V (or MEP amplitude decreases more than 30%) it is considered a warning sign of spinal cord ischemia.[
IOM can be associated to endovascular network techniques such as indocyaine green fluorescent dye (ICG) videoangiography before and after clipping. This intraoperative angiography integrated to a surgical microscope allows the surgeon to evaluate real-time images of arterial, capillary, and venous flow; thus, to recognize spinal cord ischemia even before IOM. However, this technology is not available worldwide, it is time consuming, expensive, requires additional experienced staff, and bears a complication rate of 0.4–2.6%.[
The use of epidural or intradural electrodes to measure D-wave is very useful to improve the accuracy of IOM during surgery in and around the spinal cord, but unfortunately it was not easily available in our country by the time this procedure was performed.
Surgical treatment should be considered only if connective tissue disorders, inflammatory and noninflammatory vasculopathies are excluded as the underlying etiology, mainly because under these circumstances aneurysms may thrombose or spontaneously regress with the primary pathology control.[
In cases of aneurysm rupture, prompt occlusion should be considered to remove the associated blood clot or to prevent a possible devastating re-bleeding. If there is no evidence of distal flow, the aneurysms can be obliterated with occlusion of the parent vessel.[
We describe a clear warning of possible spinal cord ischemia, within 2 minutes of temporary occlusion of the radicular artery, the threshold for MEP increased more than 100 V, and at minute 3, MEP responses vanished despite increasing the stimulus voltage.
CONCLUSIONS
This paper, attempts to build awareness of the possibility to cause or worsen a neurological deficit if a radiculomedullary aneurysm with preserved distal flow is clipped or embolized without an optimal IOM control. We report in detail the behavior of the MEPs monitoring during the occlusion of a unilateral T4 segmental artery that supplies an anterior radiculomedullary artery aneurysm.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Video Available on: www.surgicalneurologyint.com
References
1. Apel DM, Marrero G, King J, Tolo VT, Bassett GS. Avoiding paraplegia during anterior spinal surgery. The role of somatosensory evoked potential monitoring with temporary occlusion of segmental spinal arteries. Spine (Phila Pa 1976). 1991. 16: S365-70
2. Balamurugan S, Agrawal A, Kato Y, Sano H. Intra operative indocyanine green video-angiography in cerebrovascular surgery: An overview with review of literature. Asian J Neurosurg. 2011. 6: 88-93
3. Bassett G, Johnson C, Stanley P. Comparison of preoperative selective spinal angiography and somatosensory evoked potential monitoring with temporary occlusion of segmental vessels during anterior spinal surgery. Spine. 1996. 21: 1996-2000
4. Berlis A, Scheufler KM, Schmahl C, Rauer S, Götz F, Schumacher M. Solitary spinal artery aneurysms as a rare source of spinal subarachnoid hemorrhage: Potential etiology and treatment strategy. AJNR Am J Neuroradiol. 2005. 26: 405-10
5. Calancie B, Harris W, Brindle GF, Green BA, Landy HJ. Threshold-level repetitive transcranial electrical stimulation for intraoperative monitoring of central motor conduction. J Neurosurg. 2001. 95: 183-90
6. Calancie B, Harris W, Broton JG, Alexeeva N, Green BA. Threshold-level multipulse transcranial electrical stimulation (TES) of motor cortex for intraoperative monitoring of spinal motor tracts: Description of method and comparison to SEP monitoring. J Neurosurg. 1988. 88: 457-70
7. Calancie B, Morano MR. Alarm criteria for motor evoked potentials: What's wrong with the “presence or absence” approach?. Spine. 2008. 33: 406-14
8. Djindjic MY, Berenstein A, Lasjaunias P.editors. Spine and Spinal cord vascular lesions. Surgical Neuroangiography. Berlin: Springer-Verlag; 1992. p. 33-
9. Dong CC, MacDonald DB, Janusz MT. Intraoperative spinal cord monitoring during descending thoracic and thoracoabdominal aneurysm surgery. Ann Thorac Surg. 2002. 74: S1873-6
10. Faciszewski T, Winter RB, Lonstein JE, Denis F, Johnson L. The surgical and medical perioperative complications of anterior spinal fusion surgery in the thoracic and lumbar spine in adults. A review of 1223 procedures. Spine (Phila Pa 1976). 1995. 20: 1592-9
11. Fok M, Jafarzadeh F, Sancho E, Abello D, Rimmer L, Howard C. Is There Any Benefit of Neuromonitoring During Descending and Thoracoabdominal Aortic Aneurysm Repair?. Innovations (Phila). 2015. 10: 342-8
12. Gonzalez LF, Zabramski JM, Tabrizi P, Wallace RC, Massand MG, Spetzler RF. Spontaneous spinal subarachnoid hemorrhage secondary to spinal aneurysms: Diagnosis and treatment paradigm. Neurosurgery. 2005. 57: 1127-31
13. Guerit JM, Dion RA. State-of the-art of neuromonitoring for prevention of immediate and delayed paraplegia in thoracic and thoracoabdominal aorta surgery. Ann Thorac Surg. 2002. 74: S1867-9
14. Gutierrez Romero D, Batista AL, Gentric JC, Raymond J, Roy D, Weill A. Ruptured isolated spinal artery aneurysms. Report of two cases and review of the literature. Interv Neuroradiol. 2014. 20: 774-80
15. Hong MK, Hong MK, Pan WR, Wallace D, Ashton MW, Taylor GI. The angiosome territories of the spinal cord: Exploring the issue of preoperative spinal angiography. Laboratory investigation. J Neurosurg Spine. 2008. 8: 352-64
16. Jiarakongmun P, Chewitt P, Pongpech S. Ruptured anterior spinal artery aneurysm associated with coarctation of aorta. Intervent Neuroradiol. 2002. 8: 285-92
17. Kawamura S, Yoshida T, Nonoyama Y, Yamada M, Suzuki A, Yasui N. Ruptured anterior spinal artery aneurysm: A case report. Surg Neurol. 1999. 51: 608-12
18. Leung YL, Grevitt M, Henderson L, Smith J. Cord monitoring changes and segmental vessel ligation in the “at risk” cord during anterior spinal deformity surgery. Spine (Phila Pa 1976). 2005. 30: 1870-4
19. Mohsenipour I, Ortler M, Twerdy K, Schmutzhard E, Attlmayr G, Aichner F. Isolated aneurysm of a spinal radicular artery presenting as spinal subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 1994. 57: 767-8
20. Orchowski J, Bridwell KH, Lenke LG. Neurological deficit from a purely vascular etiology after unilateral vessel ligation during anterior thoracolumbar fusion of the spine. Spine (Phila Pa 1976). 2005. 30: 406-10
21. Rengachary SS, Duke DA, Tsai FY, Kragel P. Spinal arterial aneurysm: Case report. Neurosurgery. 1993. 33: 125-30
22. Santillan A, Nacarino V, Greenberg E, Riina HA, Gobin YP, Patsalides A. Vascular anatomy of the spinal cord. J Neurointerv Surg. 2012. 4: 67-74
23. Shiiya N, Yasuda K, Matsui Y, Sakuma M, Sasaki S. Spinal cord protection during thoracoabdominal aortic aneurysm repair: Results of selective reconstruction of the critical segmental arteries guided by evoked spinal cord potential monitoring. J Vasc Surg. 1995. 21: 970-5
24. Sloan TB, Jameson LC. Electrophysiologic monitoring during surgery to repair the thoraco-abdominal aorta. J Clin Neurophysiol. 2007. 24: 316-27
25. Sung TH, Leung WK, Lai BM, Khoo JL. Isolated spinal artery aneurysm: A rare culprit of subarachnoid haemorrhage. Hong Kong Med J. 2015. 21: 179-82
26. Tsirikos AI, Howitt SP, McMaster MJ. Segmental vessel ligation in patients undergoing surgery for anterior spinal deformity. J Bone Joint Surg Br. 2008. 90: 474-9
27. van Es AC, Brouwer PA, Willems PW. Management considerations in ruptured isolated radiculopial artery aneurysms. A report of two cases and literature review. Interv Neuroradiol. 2013. 19: 60-6
28. Winter RB, Lonstein JE, Denis F, Leonard AS, Garamella JJ. Paraplegia resulting from vessel ligation. Spine. 1996. 21: 1232-4
29. Wu L, Qiu Y, Ling W, Shen Q. Change pattern of somatosensory-evoked potentials after occlusion of segmental vessels: Possible indicator for spinal cord ischemia. Eur Spine J. 2006. 15: 335-40