- Neurosurgical Department, Asclepeion General Hospital of Athens, Athens, Greece
Correspondence Address:
Rovlias Aristedis
Neurosurgical Department, Asclepeion General Hospital of Athens, Athens, Greece
DOI:10.4103/sni.sni_418_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Rovlias Aristedis, Papoutsakis Dimitrios, Paidakakos Nikolaos, Blionas Alexandros. Intrathecal baclofen pump infection treated by adjunct intrareservoir teicoplanin instillation. 14-Mar-2017;8:38
How to cite this URL: Rovlias Aristedis, Papoutsakis Dimitrios, Paidakakos Nikolaos, Blionas Alexandros. Intrathecal baclofen pump infection treated by adjunct intrareservoir teicoplanin instillation. 14-Mar-2017;8:38. Available from: http://surgicalneurologyint.com/surgicalint_articles/intrathecal-baclofen-pump-infection-treated-by-adjunct-intrareservoir-teicoplanin-instillation/
Abstract
Background:The delivery of intrathecal baclofen via pumps is gaining increasing use in the management of intractable spasticity. One of the rare but devastating complications of this method is infection. In the majority of cases, removal of the device is required, despite appropriate intravenous antibiotic therapy. We report a case that highlights the use of intrareservoir teicoplanin to achieve sterilization of the infected pump system in a patient in whom removal of the pump was not an easy option.
Case Description:We describe our experience on a patient with cerebral palsy in whom Staphylococcus epidermidis pump infection developed due to contamination of the infusion reservoir during refilling procedure, which was successfully sterilized in situ by the combined use of systemic antibiotics and intrareservoir coadministration of baclofen with teicoplanin. The infection was eradicated and baclofen therapy was continued uninterrupted.
Conclusions:Removal of intrathecal baclofen pump is not necessary as the first measure in cases with mild clinical symptomatology. In view of the fact that pumps for intrathecal drug delivery are very costly, salvage of the device may be attempted in selected cases, although it is not generally recommended. Combined infusion of baclofen and an antibiotic through the pump makes it possible to maintain treatment for spasticity, sterilize the pump reservoir and flow tubes, and effectively treat infections that develop during the use of these systems.
Keywords: Infection, intrathecal baclofen pump, intrathecal teicoplanin
INTRODUCTION
Nowadays, intrathecal baclofen (ITB) administration is a widely accepted safe and effective therapy for the treatment of intractable spasticity, and its clinical efficacy has been demonstrated by several studies. ITB pumps are commonly used for the treatment of spasticity in numerous pathological processes, including spinal cord injury, multiple sclerosis, cerebral palsy, traumatic brain injury, dystonia, and stroke, particularly for patients who are unresponsive to conservative pharmacotherapy or experience intolerable side effects at therapeutic doses of oral baclofen.[
Even though technological advances have improved the efficacy and safety of this procedure, various complications related to these drug delivery systems have been described, which are usually related to catheter malfunction or pump–catheter connection problems.[
This article reports our experience in the treatment of an ITB programmable pump infection from Staphylococcus epidermidis due to contamination of the infusion pump reservoir during refiling procedure in a patient with cerebral palsy. We discuss our strategy and relevant considerations in the successful management of this complication without removal of the device via continuous intrareservoir teicoplanin administration together with baclofen.
CASE DESCRIPTION
A 34-year-old man with a medical history of cerebral palsy and spastic quadriparesis underwent an ITB Prometra programmable 20 ml pump (Flowonix Medical Inc., New Jersey, USA) implantation for spasticity refractory to high doses oral baclofen 2 years ago. ITB therapy resulted in a progressive improvement in patient's spasticity, functional capacity, daily activity, and social interactions, with a reduction in his previous disability. Three weeks ago, he had a routine refill procedure of the intrathecal pump programmed to deliver baclofen at a dose of 250 μg/day to control his spasticity.
He was admitted suffering from a pyrexia and headache, however, he had no other symptoms. On examination, he had a temperature of 38.5°C, but was not clinically toxic. He was alert, cooperative, and obeyed commands. He had no meningism and no obvious source of infection. A chest radiograph was normal. Empirical treatment with intravenous vancomycin (500 mg/6 h) and cefotaxime (1 g/12 h) was started, however, the next day vancomycin was switched to teicoplanin (400 mg/12 h) due to a “red man syndrome” onset. Microscopy and culture of the urine and three sets of blood cultures were negative. Specimens obtained by aspiration from the residual baclofen-containing reservoir and the side port of the cerebrospinal fluid (CSF) confirmed the presence of a S. epidermidis infection with high sensitivity to above antibiotics. Other CSF parameters included an elevated protein and white blood cell count. Patient's clinical condition was such that immediate removal of the device was not considered mandatory.
However, despite receiving high doses antibiotics treatment, the patient remained febrile over the next few days but still without meningism. Further aspiration and CSF culture from the access port confirmed the persistence of the S. epidermidis.
As the patient was unwilling to undergo surgery to replace the pump if the present one had to be removed, an attempt was made to sterilize the pump while in situ. Therefore, consent was obtained from both the patient and his family to maintain the implanted drug delivery system and administer a baclofen/teicoplanin solution intrathecally by using the pump that was already in place. After emptying of the reservoir and the catheter from baclofen independently, the pump was aseptically refilled with a 20-ml solution prepared containing 10 mg baclofen (5 ml), 800 mg teicoplanin (6 ml), and 9 ml normal saline. This provided a final concentration of 500 μg/ml baclofen and 40 mg/ml teicoplanin. The pump was programmed to run by simple continuous infusion at a rate of 0.5 ml of solution daily, which provided 250 μg of baclofen and 20 mg of teicoplanin intrathecally every 24 hours. Although pharmaceutical data suggests that teicoplanin is stable,[
Five days after initiation of baclofen/teicoplanin infusion, the patient became afebrile. In CSF collected from the access port, white blood cell (WBC) and protein counts were shown to have fallen significantly. On the 11th day of intrathecal coadministration of baclofen and teicoplanin, the cultures of CSF via catheter access port and of the residual fluid in the reservoir became sterile. The intravenous administration of teicoplanin and cefotaxime were discontinued, and a regimen of 600 mg rifampin administered orally per day was initiated to augment the intrathecal teicoplanin therapy, which was continued for 9 more days. The patient was treated with oral rifampin for the next month.
At the time of writing this study, our patient is still well 1 year after completion of therapy, without new clinical or laboratory signs of recurrence of infection, showing a good therapeutic reduction in his spasticity.
DISCUSSION
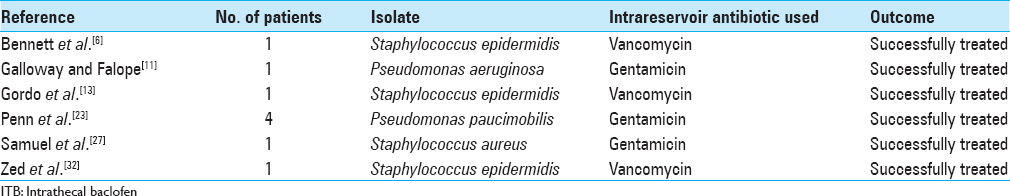
Intrathecal pumps infections are rare, mostly occurring during the first weeks following implantation, and present with fever, nuchal rigidity, and changes in the level of consciousness. The diagnosis is confirmed by positive bacterial CSF cultures, and removal of all implanted hardware and appropriate antibiotics treatment is usually necessary, especially in severe cases.[
Success rates with intravenous antibiotic agents for the treatment of pump infection have been low because of the difficulty achieving therapeutic concentrations in the CSF; therefore, intrathecal administration of antibiotic drugs is often required to eradicate the pathogen.[
To the best of our knowledge, this is the first reported case in the literature of intrathecal coadministration of baclofen and teicoplanin to successfully treat pump infection without removal of the pump or discontinuation of intrathecal baclofen. Our patient's infection, introduced during the refill procedure, was mild at presentation, and continuous infusion of baclofen with antibiotic simultaneously appeared to be the most logical treatment alternative, even though there was no information available on physical or chemical compatibility of baclofen with teicoplanin.
The total duration of intrathecal teicoplanin therapy in this patient was 3 weeks, which may have been longer than required. However, because of the possibility of an incompatibility between baclofen and teicoplanin, we believed that such a long duration therapy with teicoplanin was justified for our patient. This case report is illustrative of the safety and usefulness of long-term intrathecally administered teicoplanin.
CONCLUSIONS
The fact of considering a salvage therapy for infected ITB pumps has always been a subject of concern. Intrathecal pumps differ from other artificial implants in that direct instillation of antibiotics into the reservoir allows the antibiotic to reach the site of an infection. Thus intrareservoir antibiotics may be used to sterilize the interior of the pump, whereas systemic antibiotics combat the meningitis; therefore, an initial attempt could be made for conservative management.
This case shows another important aspect in the management of ITB pumps. The source of the infection in our patient had been probably the refill procedure 3 weeks preadmission since both aspirations from the refill port of baclofen and from the side port of CSF proved positive for S. epidermidis. Thus, a strict and meticulous asepsis during transdermal refilling is always crucial to maintain internal sterility of the system; ITB devices should be refilled as aseptically and infrequently as possible to reduce the opportunity of introducing contaminants.
Our experience indicates that, in selected patients with mild clinical symptomatology, if the treatment is offered promptly, it is possible to treat infection while maintaining treatment for spasticity control without removing the ITB device. In addition, health care insurers will not be asked to cover the increased costs for this type of therapy. Although in some countries the replacement of an expensive device may not be of immediate big concern, increasing medical costs in general are impacting negatively the health care systems, necessitating ultimately global cost containment measures and policies. A proposal of other alternatives, before removal of the pump, should be taken into account to save such a high costly device.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Albright AL, Barry MJ, Fasick P, Barron W, Shultz B. Continuous intrathecal baclofen infusion for symptomatic generalized dystonia. Neurosurgery. 1996. 38: 934-9
2. Armstrong RW, Steinbok P, Cochrane DD, Kube SD, Fife SE, Farrell K. Intrathecally administered baclofen for treatment of children with spasticity of cerebral origin. J Neurosurg. 1997. 87: 409-14
3. Awaad Y, Tayem H, Munoz S, Ham S, Michon AM, Awaad R. Functional assessment following intrathecal baclofen therapy in children with spastic cerebral palsy. J Child Neurol. 2003. 18: 26-34
4. Awaad Y, Rizk T, Siddiqui I, Roosen N, McIntosh K, Walnes GM. Complications of intrathecal baclofen pump: Prevention and cure. ISRN Neurol 2012. 2012. p. 575168-
5. Azouvi P, Mane M, Thiebaut JB, Denys P, Remy-Neris O, Bussel B. Intrathecal baclofen administration for control of severe spinal spasticity: Functional improvement and long-term follow-up. Arch Phys Med Rehabil. 1996. 77: 35-9
6. Bennett MI, Tai YMA, Symonds JM. Staphylococcal meningitis following Synchromed intrathecal pump implant: A case report. Pain. 1994. 56: 243-4
7. Coffey JR, Cahill D, Steers W, Park DS, Ordia J, Meythaler J. Intrathecal baclofen for inrtactable spasticity of spinal origin: Results of a long-term multicenter study. J Neurosurg. 1993. 78: 226-32
8. Fjelstad AB, Hommelstad J, Sorteberg A. Infections related to intrathecal baclofen therapy in children and adult: Frequency and risk factors. J Neurosurg Pediatr. 2009. 4: 487-93
9. Flückiger B, Knecht H, Grossmann S, Felleiter P. Device-related complications of long-term intrathecal drug therapy via implanted pumps. Spinal Cord. 2008. 46: 639-43
10. Follett KA, Boortz-Marx RL, Drake JM, DuPen S, Schneider SJ, Turner MS. Prevention and management of intrathecal drug delivery and spinal cord stimulation system infections. Anesthesiology. 2004. 100: 1582-94
11. Galloway A, Falope FZ. Pseudomonas aeruginosa infection in an intrathecal baclofen pump: Successful treatment with adjunct intra-reservoir gentamicin. Spinal Cord. 2000. 38: 126-8
12. Gilmartin R, Bruce D, Storrs BB, Abbott R, Krach L, Ward J. Intrathecal baclofen for management of spastic cerebral palsy: Multicenter trial. J Child Neurol. 2000. 15: 71-7
13. Gordo AH, Pérez VV, Cervelló AP, Olmedo GC, López MAP. Continuous infusion of baclofen and an antibiotic for treating meningitis related to refilling of an intrathecal infusion pump reservoir. Rev Esp Anestesiol Reanim. 2008. 55: 43-6
14. Knight KH, Brand FM, Mchaourab AS, Veneziano G. Implantable intrathecal pumps for chronic pain: Highlights and updates. Croat Med J. 2007. 48: 22-34
15. Lazorthes Y, Sallerin-Caute B, Verdie JC, Bastide R, Carillo JP. Chronic intrathecal baclofen administration for control of severe spasticity. J Neurosurg. 1990. 72: 393-402
16. Meythaler JM, McCary A, Hadley MN. Prospective assessment of continuous intrathecal infusion of baclofen for spasticity caused by acquired brain injury: A preliminary report. J Neurosurg. 1997. 87: 415-9
17. Middel B, Kuipers-Upmeijer H, Bouma J, Staal M, Oenema D, Postma T. Effect of intrathecal baclofen delivered by an implanted programmable pump on health related quality of life in patients with severe spasticity. J Neurol Neurosurg Psychiatry. 1997. 63: 204-9
18. Motta F, Buonaguro V, Stignani C. The use of intrathecal baclofen pump implants in children and adolescents: Safety and complications in 200 consecutive cases. J Neurosurg. 2007. 107: 32-5
19. Naveira FA, Speight KL, Rauck RL, Carpenter RL. Meningitis after injection of intrathecal baclofen. Anesth Analg. 1996. 82: 1297-9
20. Ooi YC, Malone J, DeVera T, Blyzniuk C, Sharan A. Successful re-implantation of intrathecal delivery system after removal secondary to infection or wound dehiscence. JHN J. 2010. p. 13-5
21. Ordia JI, Fischer E, Adamski E, Spatz EL. Chronic intrathecal delivery of baclofen by a programmable pump for the treatment of severe spasticity. J Neurosurg. 1996. 85: 452-7
22. Penn RD. Medical and surgical treatment of spasticity. Neurosurg Clin N Am. 1990. 1: 719-27
23. Penn RD. Intrathecal baclofen for spasticity of spinal origin: Seven years of experience. J Neurosurg. 1992. 77: 236-40
24. Plassat R, Perrouin Verbe B, Menei P, Menegalli D, Mathé JF, Richard I. Treatment of spasticity with intrathecal baclofen administration: Long-term follow-up, review of 40 patients. Spinal Cord. 2004. 42: 686-93
25. Purves D, Augustine GJ, Fitzpatrick D, Hall WC, LaMantia AS, McNamara JO.editors. Lower motor neuron circuits and motor control. Neuroscience. Sunderland, Mass, USA: Sinauer Publishers; 2004. p. 379-81
26. Purves D, Augustine GJ, Fitzpatrick D, Hall WC, LaMantia AS, McNamara JO.editors. Upper motor neuron control of the brainstem and spinal cord. Neuroscience. Sunderland, Mass, USA: Sinauer Publishers; 2004. p. 414-5
27. Samuel M, Finnerty GT, Rudge P. Intrathecal baclofen pump infection treated by adjunct intrareservoir antibiotic instillation. J Neurol Neurosurg Psychiatry. 1994. 57: 1146-7
28. Saval A, Chiodo AE. Intrathecal baclofen for spasticity management: A comparative analysis of spasticity of spinal vs cortical origin. J Spinal Cord Med. 2010. 33: 16-21
29. Teddy P, Jamous A, Gardner B, Wang D, Silver J. Complications of intrathecal baclofen delivery. Br J Neurosurg. 1992. 6: 115-8
30. Vender JR, Hester S, Waller JL, Rekito A, Lee MR. Identification and management of intrathecal baclofen pump complications: A comparison of pediatric and adult patients. J Neurosurg. 2006. 104: 9-15
31. Wilson AP. Clinical pharmacokinetics of teicoplanin. Clin Pharmacokinet. 2000. 39: 167-83
32. Zed PJ, Stiver HG, Devonshire V, Jewesson PJ, Marra F. Continuous intrathecal pump infusion of baclofen with antibiotic drugs for treatment of pump-associated meningitis. J Neurosurg. 2000. 92: 347-9