- REHAB Basel, Clinic for Neurorehabilitation and Paraplegiology, Basel, Switzerland
- Department of Neurosurgery, Bethesda Hospital, Basel, Switzerland
Correspondence Address:
Christian Saleh
REHAB Basel, Clinic for Neurorehabilitation and Paraplegiology, Basel, Switzerland
DOI:10.25259/SNI-33-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Moritz Haering, Christian Saleh, Phillip Jaszczuk, Markus Koehler, Margret Hund-Georgiadis. Intrathecal pump catheter-tip granuloma recurrence with associated myelomalacia – How safe is intrathecal analgesic infusion therapy? A case report. 24-Apr-2019;10:62
How to cite this URL: Moritz Haering, Christian Saleh, Phillip Jaszczuk, Markus Koehler, Margret Hund-Georgiadis. Intrathecal pump catheter-tip granuloma recurrence with associated myelomalacia – How safe is intrathecal analgesic infusion therapy? A case report. 24-Apr-2019;10:62. Available from: http://surgicalneurologyint.com/surgicalint-articles/9292/
Abstract
Background:A serious complication of intrathecal (IT) infusion therapy for pain management is catheter-tip-associated granuloma. Catheter-tip granulomas can lead to permanent severe neurological sequelae if not promptly detected.
Case Description:We report a patient with a recurrence of a catheter-tip granuloma causing a high-grade paresis of the lower extremities and we review briefly the literature.
Conclusion:Patients with IT pump therapy presenting new neurological findings need prompt imaging of the spinal axis to rule out a catheter-tip granuloma. In case of catheter-tip granuloma, early surgical decompression is important.
Keywords: Catheter-tip granuloma, Complications, Intrathecal pump therapy
INTRODUCTION
Invasive nonmalignant pain management with intrathecal (IT) analgesic infusion therapy is a common strategy for the treatment of “failed back surgery syndrome” (FBSS). Catheter-tip-associated granulomas are a known but rare complication of IT analgesic infusion therapy with potentially severe neurological consequences occurring in <3% of morphine pump patients.[
IT drug therapy is known to be an effective treatment method for patients with FBSS.[
An utmost serious and underestimated adverse effect of the central route is the formation of a catheter-tip-associated granuloma.[
CASE PRESENTATION
A 75-year-old female patient with a history of multiple spine surgeries, chronic pain syndrome (cervicalgia and lumbalgia), and a slightly ataxic gait was treated with IT morphine and clonidine infusion (SynchroMed II Pump Medtronic) since 2012. In April 2018, she presented with new-onset paresthesia sub-T5. A magnetic resonance imaging (MRI) of the spine showed high-grade compression of the spinal cord (with compression-induced myelomalacia) at T3–T5 corresponding to the location of the catheter tip with suspicion of a catheter-tip-associated granuloma [Figures
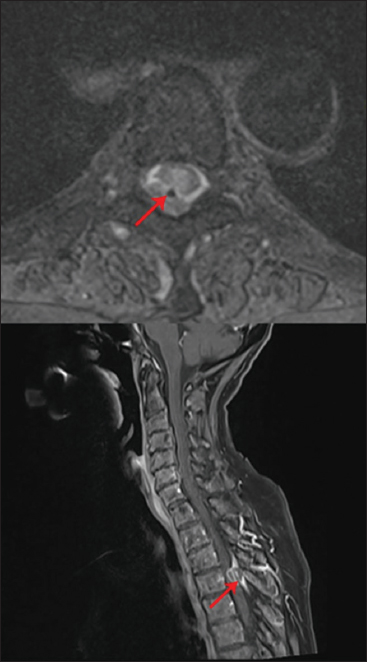
Figure 1
Upper image: T2 axial image of the lesion surrounding the catheter (red arrow). The granuloma appears as an extra-axial lesion isodense to the myelon. Lower image: T1 contrast sagittal image showing a space-occupying, ring-enhancing, inhomogeneous, extra-axial mass (red arrow) in the spinal canal at the level of T4.
A right-sided hemilaminectomy of T4 as well as a partial hemilaminectomy of T3 and T5 was performed. The exposed dura was thickened and adherent to the surrounding structures. After removal of the adhesions and preparation of the dura, a necrotic-seeming central thickening, continuous with the surrounding normal dura, was identified as the granulomatous mass. This highly adherent mass was carefully resected along a clearly defined plane between the granuloma and the normal dura. A partial resection of the healthy dura surrounding the right T4 nerve root was required. The catheter was then identified inside the spinal canal and removed. Due to adherence to the T4 nerve root, the decision was made to sacrifice the T4 nerve. The granuloma was then carefully resected along with the divided nerve. The underlying spinal cord had signs of metal deposits from the catheter as well as signs of myelomalacia. The spinal cord was completely decompressed, and the primary closure of the dura was possible.
Postsurgery, the patient showed a moderate- to high-grade proximally pronounced paresis of the right lower extremity, which slightly improved in the days following surgery. MRI revealed a satisfactory decompression of the spinal cord and removal of the granuloma. However, the MRI showed a more marked (preexisting) myelomalacia extending now from T2 to T7. Additional oral analgesia oxycodone 5 mg twice daily and trimipramine 100 mg once daily had to be continued unchanged, for the persistence of foremost lumbar pain. After an initial stable clinical course, she complained of increasing neuropathic pain initially located mostly in the T9–T12 area and subsequently ascending into the thorax approximately 2 months after admission. Pain medication had to be increased by adding pregabalin and cannabis oil (drops) and increasing the morphine and clonidine pump dosage yet without clinical benefit. Clinically, the patient complained of instability and sensory loss in the left leg. A lumbar MRI was performed, which did not explain the new findings. Within 1 week, the patient deteriorated dramatically loosing almost all motor strength in the lower extremities. A cervical and thoracic MRI [
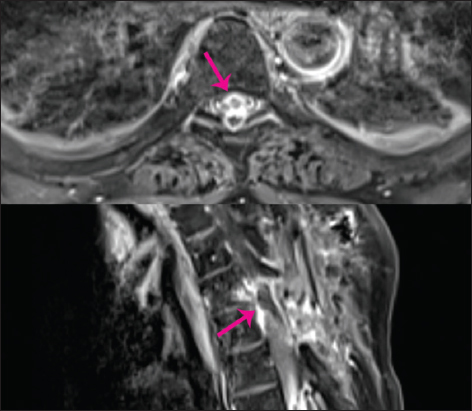
Figure 3
T1 contrast imaging. Upper image: Axial image showing a large recurrence overshadowing and compressing the spinal cord (pink arrow). Lower image: The recurrent granuloma is seen here as a large, ring-enhancing, inhomogeneous, mass-causing high-grade compression of the spinal cord (pink arrow).
A laminectomy of T6 was performed. The underlying tissue was granulomatous, and the catheter was identified within the granulomatous mass. The lesion was excised leaving a dural defect that could not be repaired with primary closure. Watertight closure with a Gore-Tex patch was achieved. The remaining catheter was removed through a separate incision, and a new catheter was not placed.
The histopathological finding showed typical granuloma tissue formed by histiocytes, granulocytes, necrotic areas, and abundant hemorrhagic residues. The neurological state of the patient improved mildly after device removal, granuloma excision, and intense rehabilitation foremost gaining slight-to-moderate motor strength in the left leg.
DISCUSSION
The literature describes several cases of IT pump catheter-tip-associated granuloma (CG), a phenomenon possibly due to an inflammatory response to the infused drug or a foreign-body reaction to biomaterials.[
Granuloma recurrence
Granuloma recurrence is rarely described in the literature. The first report on granuloma recurrence was reported by Hoederath et al.[
Granuloma formation with analgesics
Deer et al.[
CONCLUSION
CGs are rare but serious complications of IT infusion treatment as they can lead to permanent neurological sequelae. As our case demonstrates, diagnosis of granuloma occurrence in this pool of patients risks to be delayed considering the multimorbidity of these patients. New or worsening symptoms might be attributed initially to other conditions. Therefore, patients with IT showing neurologic symptoms should obtain prompt spine imaging to rule out a spinal cord-compressing granuloma. Asymptomatic patients with a history of granuloma should obtain regular spine MRI to rule out a recurrence. From the few reports and from our case, it appears that recurrence occurs within a brief time period of approximately 6 months. CG cases need rigorous reporting to be able to estimate accurately the benefit–risk ratio of IT therapy in pain management.
Take-home points
IT pump dysfunction should always be suspected if the patient is unresponsive to increasing drug dosages. Morphine seems to be associated with the highest risk of granuloma formation. All patients with IT pump therapy showing new neurological findings need prompt imaging of the whole spinal axis to rule out a catheter-tip granuloma. Asymptomatic patients with a history of catheter-tip granuloma need regular spine MRI as granuloma recurrences appear to occur within months of the preceding granuloma. Catheter-tip granuloma diagnosis must be followed by prompt surgical decompression.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that her name and initials will not be published and due efforts will be made to conceal her identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Anderson JM, Rodriguez A, Chang DTForeign body reaction to biomaterials Semin Immunol. 2008. 20: 86-100
2. Arnold PM, Harsh V, Oliphant SM. Spinal cord compression secondary to intrathecal catheter-induced granuloma:A report of four cases. Evid Based Spine Care J. 2011. 2: 57-62
3. Bottros MM, Christo PJ. Current perspectives on intrathecal drug delivery. J Pain Res. 2014. 7: 615-26
4. De Andres J, Palmisani S, Perez VL, Asensio J, Lopez-Alarcon MD. Can an intrathecal, catheter-tip-associated inflammatory mass reoccur?. Clin J Pain. 2010. 26: 631-4
5. Decramer T, Morlion B, van Calenbergh F, Nuttin B, van Loon J, Theys T. Unexpected symptomatic catheter tip mass in chronic intrathecal opioid therapy. Pain Med. 2016. 17: 1571-3
6. Deer TR. A prospective analysis of intrathecal granuloma in chronic pain patients:A review of the literature and report of a surveillance study. Pain Phys. 2004. 7: 225-8
7. Deer TR, Prager J, Levy R, Rathmell J, Buchser E, Burton A. Polyanalgesic consensus conference--2012:Consensus on diagnosis, detection, and treatment of catheter-tip granulomas (inflammatory masses). Neuromodulation. 2012. 15: 483-95
8. Deer TR, Raso LJ, Garten TG. Inflammatory mass of an intrathecal catheter in patients receiving baclofen as a sole agent:A report of two cases and a review of the identification and treatment of the complication. Pain Med. 2007. 8: 259-62
9. Gupta A, Martindale T, Christo PJ. Intrathecal catheter granuloma associated with continuous sufentanil infusion. Pain Med. 2010. 11: 847-52
10. Hoederath P, Gautschi OP, Land M, Hildebrandt G, Fournier JY. Formation of two consecutive intrathecal catheter tip granulomas within nine months. Cent Eur Neurosurg. 2010. 71: 39-42
11. Kratzsch T, Stienen MN, Reck T, Hildebrandt G, Hoederath P. Catheter-tip granulomas associated with intrathecal drug delivery a two-center experience identifying 13 cases. Pain Phys. 2015. 18: E831-40
12. Narouze SN, Casanova J, Souzdalnitski D. Patients with a history of spine surgery or spinal injury may have a higher chance of intrathecal catheter granuloma formation. Pain Pract. 2014. 14: 57-63
13. North RB, Cutchis PN, Epstein JA, Long DM. Spinal cord compression complicating subarachnoid infusion of morphine:Case report and laboratory experience. Neurosurgery. 1991. 29: 778-84
14. Phillips JA, Escott EJ, Moossy JJ, Kellermier HC. Imaging appearance of intrathecal catheteter tip granulomas:Report of three cases and review of the literature. AJR. 2007. 189: 375-81






Joe Ordia
Posted May 6, 2019, 6:54 pm
I applaud the authors for drawing attention to one of the most serious potential complications of IT analgesic infusion pumps.
The authors are correct that IT morphine has been associated with many of the reported cases of catheter tip granuloma (CS). It must also be mentioned that patients on high concentrations of morphine are at a higher risk.
The patient was on IT morphine and clonidine; what was the concentration of morphine?
Was any thought given to switching from morphine to another analgesic that is less likely to result in CG?
Dr. Christian Saleh
Posted May 15, 2019, 9:19 am
We thank Dr. Ordia for his comments.
The initial clonidine dosage was 0.24151 mg/day at a concentration of 0.3 mg/ml, which was increased subsequently to a dosage of 0.25390 mg/day maintaining the concentration at 0.3 mg/ml. For morphine the initial dosage was 27.371 mg/day at a concentration of 34 mg/ml. The morphine dosage was subsequently increased to 28.776 mg/day maintaining the concentration at 34 mg/ml. We had planned a medication switch to fentanyl, but this was not carried out due to the complications described in our article that required a surgical approach.
With best wishes and on behalf of all authors,
Christian Saleh