- Department of Neurosurgery, Barrow Neurological Institute, St. Joseph's Hospital and Medical Center, Phoenix, Arizona, USA
- Department of Neurosurgery, Irkutsk State Medical University, Irkutsk, Russia
- University of Arizona College of Medicine, Tucson, Arizona, USA
- School of Life Sciences, Arizona State University, Tempe, Arizona, USA
Correspondence Address:
Mark C. Preul
Department of Neurosurgery, Barrow Neurological Institute, St. Joseph's Hospital and Medical Center, Phoenix, Arizona, USA
DOI:10.4103/sni.sni_489_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Evgenii Belykh, Kaan Yagmurlu, Nikolay L. Martirosyan, Ting Lei, Mohammadhassan Izadyyazdanabadi, Kashif M. Malik, Vadim A. Byvaltsev, Peter Nakaji, Mark C. Preul. Laser application in neurosurgery. 09-Nov-2017;8:274
How to cite this URL: Evgenii Belykh, Kaan Yagmurlu, Nikolay L. Martirosyan, Ting Lei, Mohammadhassan Izadyyazdanabadi, Kashif M. Malik, Vadim A. Byvaltsev, Peter Nakaji, Mark C. Preul. Laser application in neurosurgery. 09-Nov-2017;8:274. Available from: http://surgicalneurologyint.com/?post_type=surgicalint_articles&p=8667
Abstract
Background:Technological innovations based on light amplification created by stimulated emission of radiation (LASER) have been used extensively in the field of neurosurgery.
Methods:We reviewed the medical literature to identify current laser-based technological applications for surgical, diagnostic, and therapeutic uses in neurosurgery.
Results:Surgical applications of laser technology reported in the literature include percutaneous laser ablation of brain tissue, the use of surgical lasers in open and endoscopic cranial surgeries, laser-assisted microanastomosis, and photodynamic therapy for brain tumors. Laser systems are also used for intervertebral disk degeneration treatment, therapeutic applications of laser energy for transcranial laser therapy and nerve regeneration, and novel diagnostic laser-based technologies (e.g., laser scanning endomicroscopy and Raman spectroscopy) that are used for interrogation of pathological tissue.
Conclusion:Despite controversy over the use of lasers for treatment, the surgical application of lasers for minimally invasive procedures shows promising results and merits further investigation. Laser-based microscopy imaging devices have been developed and miniaturized to be used intraoperatively for rapid pathological diagnosis. The multitude of ways that lasers are used in neurosurgery and in related neuroclinical situations is a testament to the technological advancements and practicality of laser science.
Keywords: Laser, laser-induced thermal therapy, neurosurgery, photodynamic therapy, transcranial laser therapy
INTRODUCTION
Investigation of medical applications of lasers began shortly after their creation in 1960. Even the earliest studies suggested great therapeutic value in this newfound technology.[
In the present paper, we review the current technology and application of lasers in neurosurgery. The topics covered include magnetic resonance (MR)-guided percutaneous ablation, anastomosis, therapeutic applications, and diagnostic applications [
SURGICAL LASERS
Lasers in open surgery
Since the first use of a laser in human patients with malignant gliomas was reported in 1966,[
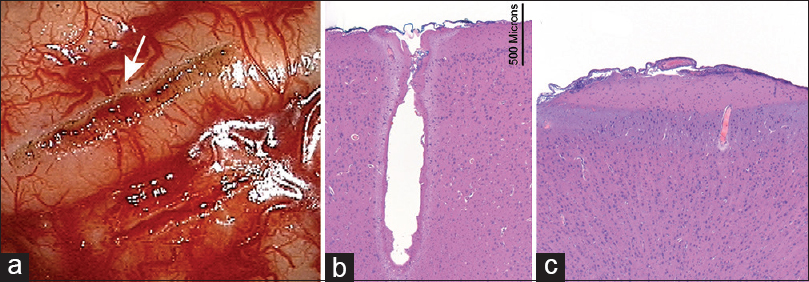
Figure 1
(a) Gross appearance of pial incisions made by a 7 W CO2 laser (top, arrow) and with bipolar microscissors (bottom) (in vivo experiment, porcine brain). (b) Hematoxylin-and-eosin (H and E) stain shows a deep laser cut through the brain without extensive peripheral damage. Three zones of effect are visible: vaporized crater, desiccated zone, and edematous zone. The transition of the effect is rapid. (c) H and E stain of a drain shows the effect of bipolar coagulation. Two zones of effect are visible (desiccated and edematous) with no pial incision visible. Used with permission from Barrow Neurological Institute, Phoenix, Arizona
Laser-assisted endoscopic neurosurgery
Using lasers to treat arachnoid cysts has provided good results. Choi et al.[
Laser-assisted microanastomosis
Excimer laser-assisted nonocclusive anastomosis (ELANA; Elana, Inc., Columbia, Maryland, USA) is a technology used to create a high-flow bypass for cerebral revascularization.[
One of the earliest clinical research studies to assess intraoperative flow through the ELANA system was performed by van der Zwan et al.,[
A sutureless ELANA technique (SELANA) was subsequently developed and is being tested. Its main improvement over ELANA is that it uses a special metal ring that is first mounted on the end of the graft vessel and then attached to the recipient vessel tightly with two pins. The anastomosis is then sealed by a circumferential layer of BioGlue surgical adhesive (CryoLife, Inc., Kennesaw, Georgia, USA) before an arteriotomy is created by the laser as in the ELANA technique.[
The ELANA system appears to be a safe and effective tool in intracranial bypass surgery. However, despite potential benefits of nonocclusive anastomoses, cerebrovascular high-flow bypasses are still relatively uncommon procedures used in select patients and most surgeries are still performed with manual suturing of the anastomosis.[
LASER-INDUCED THERMAL THERAPY
The goal of laser-induced thermal therapy (LITT) (also referred to as laser interstitial thermal therapy, percutaneous laser ablation, laser heat ablation, MRI-guided laser surgery, and MRI-guided percutaneous laser ablation) is to achieve selective thermal injury of pathological tissue while maintaining a sharp thermal border between the tumor and normal brain tissues.[
Current systems use cooling with a constant stream of cooled water or CO2 to prevent carbonization and adhesions to the probe, thus providing smooth energy distribution around the probe. LITT is initiated by the neurosurgeon and stopped manually or automatically if any monitored temperature limits are exceeded.[
The need to preserve a sharp border between surrounding normal brain tissue and the area of ablation necessitates the use of wavelengths for rapid energy deposition and high absorption by the lesion.[
Currently available systems
The three main components of the LITT system include a flexible laser probe for transmission of laser light, a laser emitter, and an MRI-compatible head-fixation frame. A software platform with a monitor displays the estimate of predicted cell death and real MRI thermal-dose contours. After the probe is inserted in the operating room, the thermal ablation procedure is performed in the MRI suite. Thereafter, the patient is moved back into the operating room for probe removal. Alternatively, the whole procedure could be performed under intraoperative MRI monitoring. Correlation among the preoperative tumor volume, postoperative ablated volume, intraoperative critical temperature volume, and intraoperative critical dose volume is essential to assess the accuracy of LITT.
NeuroBlate system
The NeuroBlate system (Monteris Medical Corp., Plymouth, Minnesota, USA) incorporates an Nd: YAG laser that produces light transmitted through a laser-delivery probe. The directional gas-cooled probe decreases the coagulation effect near the tip of the probe, allowing more even energy penetration into the brain tissue. The tip of the probe also contains a thermocouple to monitor temperature. The laser-delivery probe is positioned by using stereotactic navigation frames and connects to the robotic probe driver.
The workstation is located in the MRI control room. The surgeon creates a procedure plan using NeuroBlate system software to control a robotic mechanism for laser depth and rotation. This remote manipulation allows repetitive ablations in different positions until the planned thermal dose reaches the desired boundaries. A 3D display provides the opportunity for multiplane assessment and better visualization of thermal therapy zones and surrounding structures.
This system received U.S. Food and Drug Administration (FDA) 510(k) clearance for planning, monitoring under MRI visualization, and use of 1,064-nm lasers to ablate, necrotize, or coagulate soft tissue through interstitial irradiation or thermal therapy in neurosurgery. So far, more than 300 procedures have been performed with this system in the United States and the results have yet to be published (ClinicalTrials.gov NCT02389855). Twelve-month results from 1,000 patients are expected by 2020 (ClinicalTrials.gov NCT02392078).
Visualase system
The Visualase system (Medtronic, plc., Dublin, Ireland) is an integrated, MRI-guided, minimally invasive laser-ablation system. The ablation system comprises a 15-W, 980-nm diode laser, flexible fiber-optic probe, and 17-gauge (1.65-mm diameter) internally cooled catheter.[
The system is connected to a computer workstation in the MRI unit, which allows the display of real-time thermographic data at the treatment site.[
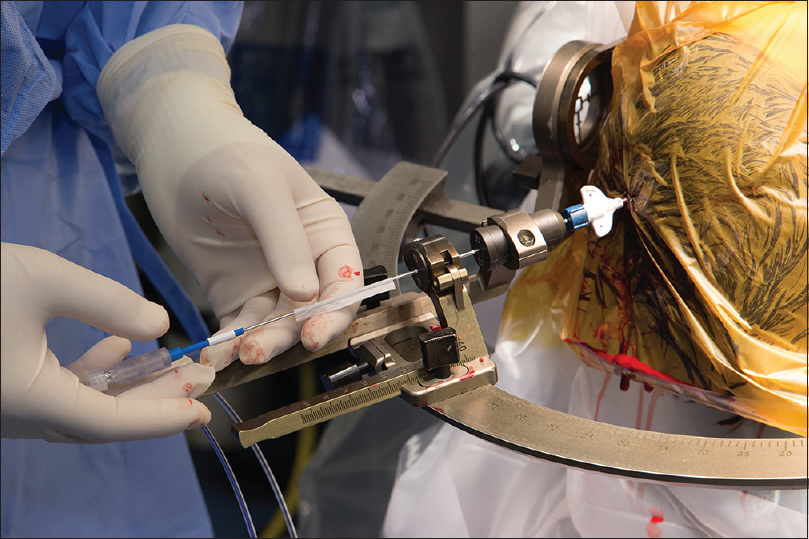
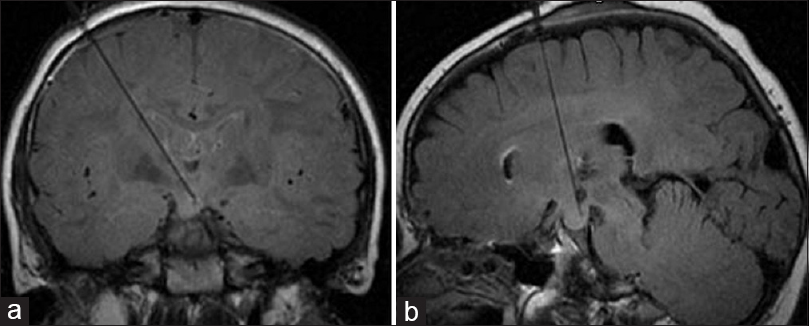
Figure 4
Visualase laser workstation setup in the magnetic resonance imaging (MRI) control room during a procedure. Monitor shows actual MRI thermal map image and predicted zone of damage, automatically calculated based on the temperature and duration of treatment. The laser source is located on the lower rack of the workstation. The blue cord is a laser fiber that transports the laser light to the patient inside the MRI machine. Used with permission from Barrow Neurological Institute, Phoenix, Arizona
The neurosurgeon controls the probe position manually inside the MRI and regulates ablation time and intensity on the workstation. The ablation may be intentionally stopped by the surgeon or automatically stopped by the system when the planned ablation has been achieved or when measured temperatures at specifically predetermined voxels exceed the operator-defined threshold. After ablation, the tissue is given time to cool to prevent carbonization and vaporization, and ablation may then be repeated in the same or a different location, producing a sausage-shaped area of damage.
Surgical applications
Lesional epilepsy
MRI-guided laser thermal ablation for epilepsy is emerging as a technique to treat a variety of epileptogenic foci, such as hypothalamic hamartomas, cortical dysplasias, cortical malformations, or the amygdalohippocampal complex. Visualase laser probe positioning by the robotic stereotactic assistant, ROSA (Medtech SA, France), with MR-guided thermal ablation for medically refractory epilepsy, has been successful.[
Hypothalamic hamartomas
Hypothalamic hamartomas are a rare type of non-neoplastic lesion located on the floor of the third ventricle. They are more common in children, and patients may present with gelastic seizures, precocious puberty, hormone imbalances, cognitive impairment, behavioral problems, and emotional difficulties. The indications for more invasive treatment options arise because of intolerance to, or ineffectiveness of, conservative therapy, most frequently caused by side effects of antiseizure therapy. Surgical resection and Gamma Knife (Elekta AB, Stockholm, Sweden) radiotherapy are possible options but carry a high risk of damage to adjacent eloquent brain structures. Therefore, laser ablation is an attractive alternative for such patients. During a single procedure, laser ablation can be stereotactically delivered with high accuracy to the place where the hamartoma attaches to the brain tissue, thus disconnecting the abnormal firing neurons from the normal tissue [
In general, MR-guided laser thermal ablation holds significant promise as a minimally invasive alternative for surgical treatment of brain epileptogenic foci. However, its long-term efficacy is unknown. Evidence is forthcoming as more centers conduct clinical trials of this new technology (e.g., ClinicalTrials.gov NCT01703143) and begin to adopt it.[
Brain metastases
Laser treatment has been successfully applied to cerebral metastases. This approach could be applied for lesions less than 3 cm in diameter, even those with elongated shapes, and especially for radioresistant lesions. However, one study was terminated due to slow accrual (ClinicalTrials.gov NCT00720837). Initially, tumor volume increases after the procedure, but over time, tumor volume decreases and may progress to complete absence in most cases.[
Ependymomas
Ependymomas are a relatively radiosensitive but chemoresistant type of brain tumor, which usually progresses if complete resection of the tumor cannot be accomplished. The standard management approach for ependymomas is surgery followed by radiotherapy, depending on tumor grade.[
Another potential limitation of LITT in intraventricular or paraventricular lesions is the presence of cerebrospinal fluid neighboring the ablation site, which likely acts as a heat sink preventing the spread of thermal energy.[
Gliomas
MRI-guided LITT may be implemented in patients with glioblastoma multiforme (GBM) who would otherwise undergo biopsy only.[
In a Phase I trial, adult patients with recurrent or progressive GBM in whom standard therapy (radiotherapy with or without chemotherapy) failed were candidates for NeuroBlate MRI-guided LITT and were followed for a minimum of 6 months or until death.[
Carpentier et al.[
Another possible use of MRI-guided LITT is disruption of the peritumoral blood–brain barrier to enhance drug delivery and efficacy for treatment of pediatric brain tumors. This use is under investigation in a phase 0 trial (ClinicalTrials.gov NCT02372409). Phase 1 and 2 trials to investigate a synergetic effect with MK-3475, a molecule that was designed to restore the natural ability of the immune system to recognize and target cancer cells, are also underway (ClinicalTrials.gov NCT02311582). Results of a pilot study of LITT and doxorubicin hydrochloride in treating patients with recurrent glioblastoma are also awaited (ClinicalTrials.gov NCT01851733).
Radiation necrosis
Radiation necrosis develops months to years after intracranial radiotherapy and is difficult to distinguish from tumor recurrence on standard MRI.[
Safety and limitations of LITT
Specific risks of LITT include damage to the cerebral vasculature by the laser probe, which could result in hemorrhage or pseudoaneurysm that may require subsequent open or endovascular surgery. Although MR thermometry allows precise control of the ablated tissue, the risk of damage to the critical cortex areas and white matter tracts by the probe or thermal energy remains.[
Nonspecific adverse effects include balance disorder, dizziness, and headache. Brain abscess, seizures, and wound infection have also been reported after LITT.[
LASER PHOTODYNAMIC THERAPY FOR BRAIN TUMORS
Laser photodynamic therapy (PDT) uses a photosensitizer that accumulates in the tumor. After illumination with a light source at the photosensitizer's absorption wavelength, the photosensitizer excites to a singlet state of higher energy. The relaxation from an excited state to a ground state after emission of a fluorescent photon generates singlet oxygen, which leads to mitochondrial and nuclear DNA damage and cell death.[
Various compounds such as porphyrin, m-tetrahydroxyphenylchlorin (temoporfin), 5-aminolevulinic acid (5-ALA), boronated porphyrin, and talaporfin sodium have been used as photosensitizers administered orally, intravenously, or locally in clinical and in-vitro trials for gliomas.[
A principal requirement for efficacious PDT of brain tumors is to achieve adequate light illumination throughout the targeted tissue volume.[
Several strategies have been proposed for even dispersion of the light. Standard approved PDT uses cylindrical diffusing fiber tips stereotactically placed for interstitial irradiation,[
In glioma surgery, several PDT approaches have been used:[
Safety
Side effects of PDT are usually related to the sensitization of the skin to light and brain edema. Other adverse events are solely related to the surgical procedure. Laser-related potential risks include brain edema, hyperthermia injury, hemorrhages, and thrombus formation.[
LASERS IN SPINAL NEUROSURGERY
Percutaneous laser disk decompression
Degeneration of intervertebral disks and disk herniation are common causes of low-back pain and sciatica, affecting more than 80% of the population.[
Indications
Laser diskectomy has been shown to be beneficial in patients suffering from a single-level disk herniation with associated radicular pain refractory to conservative treatment. Optimal candidates are patients with limited bulging of the disk herniation.
Contraindications include previous surgery at the same disk level, spinal stenosis, disk fragmentation, and migration with significant neurological deficits due to herniation. However, Choy et al.[
Currently used lasers
Nd: YAG laser
In the mid-1980s, Choy et al.[
KTP laser
Early findings by Davis et al.[
Ho: YAG laser
A Ho:YAG laser has mid-infrared wavelengths and is used in a pulsed mode to ablate the nucleus of the disk. The laser produces 1.6 J of energy per pulse with a pulse width of 250 microseconds at 10 Hz. Treating the disk with a pulsed laser, in comparison to continuous exposure, is assumed to provide no temperature rise in adjacent tissue, thus minimizing any harmful effects to normal tissue. The Ho:YAG laser is considered to be safe and effective, and it is FDA approved.
Safety
PLDD is considered a low-risk treatment option. The major potential complications associated with PLDD are nerve root injury from needle insertion or laser heat, or infectious spondylodiskitis attributed to nonsterile technique. Choy et al.[
Epiduroscopic laser neural decompression
Epiduroscopic laser neural decompression (ELND) is a new method for diagnosing and treating herniated disks, spinal stenosis, failed back surgery syndrome, and chronic refractory low-back pain. The technology utilizes an epiduroscope inserted in the sacral hiatus and passed into the epidural space with the patient under local anesthesia. The procedure is performed under fluoroscopic guidance through a Tuohy needle and the epiduroscope itself is approximately 3 mm in diameter (4007 Epiduroscopy Introducer Set, Myelotec, Roswell, Georgia, USA) and has dual working channels.[
LASER TISSUE SOLDERING FOR DURAL RECONSTRUCTION
Dural repair is a basic requirement for most neurosurgical procedures. Conventionally, dural reconstruction is achieved with sutures and fibrin glue. The laser tissue-soldering technique is a new alternative for dural reconstruction. The technique involves applying a soldering material (e.g. albumin) to the edges of the dura and binding the tissue using laser energy. The heat generated from the laser creates a bond between the soldering material and the dura. Results from studies comparing the efficacy of laser soldering with traditional dural closure are inconsistent. One of the earlier studies using a diode laser to repair dural lacerations in rat models reported desiccation of brain tissue.[
THERAPEUTIC APPLICATIONS OF LASERS IN NEUROSURGERY
Nerve regeneration
Mester et al. were among the first to document the medical application of lasers by reporting in 1968 the ability of a helium-neon laser to increase hair growth[
Rochkind et al.[
Transcranial laser therapy
Transcranial laser therapy (TLT) involves passing near-infrared light through the scalp and skull, with a small percentage reaching the cortex. Studies showed that TLT modulates brain function in a neurotherapeutic manner.[
TLT treatment of traumatic brain injuries has also been suggested. Oron et al.[
TLT for neurodegenerative diseases is a relatively new area of research. TLT has been shown to upregulate brain-derived neurotrophic factor expression and decrease cell loss in a transgenic mouse model for Alzheimer's disease.[
The discovery of LEDs as another source for light therapy comparable to laser therapy has further challenged the value of lasers in medical therapy. LEDs are remarkably cheaper than lasers and create a light with broader output peaks (less monochromatic); thus, a comparison of irradiation sources for therapy continues.
DIAGNOSTIC APPLICATIONS OF LASERS IN NEUROSURGERY
Neurosurgical laser-based endomicroscopy
Laser-scanning confocal microscopy (LSCM) has gained popularity in basic science research and has been applied intraoperatively for neurosurgery.[
The attachment of LSCM to endoscopes created laser-scanning confocal endomicroscopy (LSCE), which has a wide range of imaging applications in the medical sciences.[
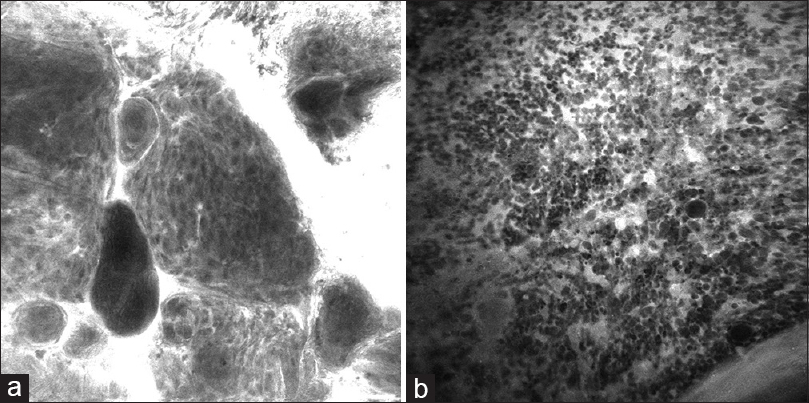
Figure 7
Images acquired by an OptiScan (OptiScan Pty. Ltd., Victoria, Australia) intraoperative confocal laser microscope with a 488-nm wavelength from brain tumor specimens treated with a fluorescein sodium dye show the clear differentiation of the cell pattern of (a) meningioma (psammoma bodies and whorling pattern) and (b) glioblastoma (multiple irregular cells with areas of necrosis). Used with permission from Barrow Neurological Institute, Phoenix, Arizona
Current systems
OptiScan
The OptiScan system (OptiScan Pty. Ltd., Victoria, Australia, and Carl Zeiss Surgical GmbH, Oberkochen, Germany) is an endoscope-integrated system in which a miniaturized confocal microscope has been placed at the distal end of a conventional endoscope. The miniaturized confocal microscope is connected to optical and personal computer units, and it uses a distal scanner with a single optical fiber. The laser emits a wavelength of 488 nm for excitation with a maximum power of 1 mW.[
Cellvizio
The Cellvizio LSCE (Mauna Kea Technologies, SA, Paris, France) is a compact maneuverable endomicroscopy system that permits multiple miniprobes to be inserted into the endoscope.[
Raman spectroscopy
Raman spectroscopy is a method of spectroscopic analysis that involves laser light interaction with molecular vibrations, phonons, or other excitations. Krafft et al.[
A subsequent study for exploring the biochemical differences between necrosis and viable tissue had 100% accuracy on 9 test patients. In a recent study, a handheld contact Raman spectroscopy probe that was developed for in-vivo local detection of cancer cells in the human brain had 93% sensitivity and 91% specificity.[
Intraoperative cerebral blood flow measurement
The capacity to assess cortical cerebral blood flow (CBF) has clinical value in neurosurgery. Three main blood flow assessment techniques, laser Doppler flowmetry (LDF), laser Doppler imaging (LDI), and laser speckle imaging (LSI) all use lasers as a source of coherent light.
LDF involves illuminating a tissue sample with a single-frequency light and measuring the frequency of backscattered light to estimate blood perfusion. This technique relies on the principle that light particles undergo a frequency or wavelength change (Doppler effect) after encountering moving red blood cells that can be detected by a sensor. A 5-mW laser diode is used in LDF with an emission wavelength of 780 nm.[
LDI uses the same theoretical framework as LDF but it provides spatially resolved CBF images by measuring the change in frequency of backscattered light at multiple points.[
LSI generates CBF information from interference patterns produced by coherent light scattering due to moving red blood cells. Real-time CBF mapping is generated from a time-varying speckle pattern that is dependent on flow speed.[
CONCLUSION
Despite early skepticism by the medical community, the application of lasers in the neurosciences, in particular, shows promising results and merits further investigation. The multitude of ways that lasers are being used in neurosurgery and in related neuroclinical situations is a testament to the technological advancements and practicality of laser science.
Financial support and sponsorship
This work was supported in part by funds from the Barrow Neurological Foundation, by funds from the Women's Board of Barrow Neurological Foundation, and by the Newsome Chair in Neurosurgery Research held by Dr. Preul.
Conflicts of interest
There are no conflicts of interest.
References
1. Ahrar K, Gowda A, Javadi S, Borne A, Fox M, McNichols R. Preclinical assessment of a 980-nm diode laser ablation system in a large animal tumor model. J Vasc Intervent Radiol. 2010. 21: 555-61
2. Akimoto J, Haraoka J, Aizawa K. Preliminary clinical report on safety and efficacy of photodynamic therapy using talaporfin sodium for malignant gliomas. Photodiagnosis Photodyn Ther. 2012. 9: 91-9
3. Ances BM, Greenberg JH, Detre JA. Laser Doppler imaging of activation-flow coupling in the rat somatosensory cortex. Neuroimage. 1999. 10: 716-23
4. Baker ML, Marino Larsen EK, Kuller LH, Klein R, Klein BE, Siscovick DS. Retinal microvascular signs, cognitive function, and dementia in older persons: The Cardiovascular Health Study. Stroke. 2007. 38: 2041-7
5. Barbosa RI, Marcolino AM, de Jesus Guirro RR, Mazzer N, Barbieri CH, de Cassia Registro Fonseca M. Comparative effects of wavelengths of low-power laser in regeneration of sciatic nerve in rats following crushing lesion. Lasers Med Sci. 2010. 25: 423-30
6. Baxter GD.editorsTherapeutic Lasers: Theory and Practice. London: Churchill Livingstone; 1994. p.
7. Beck TJ, Kreth FW, Beyer W, Mehrkens JH, Obermeier A, Stepp H. Interstitial photodynamic therapy of nonresectable malignant glioma recurrences using 5-aminolevulinic acid induced protoporphyrin IX. Lasers Surg Med. 2007. 39: 386-93
8. Belykh E, Martirosyan NL, Yagmurlu K, Miller EJ, Eschbacher JM, Izadyyazdanabadi M. Intraoperative fluorescence imaging for personalized brain tumor resection: Current state and future directions. Front Surg. 2016. 3: 55-
9. Bown SG. Phototherapy in tumors. World J Surg. 1983. 7: 700-9
10. Breen MS, Breen M, Butts K, Chen L, Saidel GM, Wilson DL. MRI-guided thermal ablation therapy: Model and parameter estimates to predict cell death from MR thermometry images. Ann Biomed Eng. 2007. 35: 1391-403
11. Briers JD. Laser Doppler, speckle and related techniques for blood perfusion mapping and imaging. Physiol Meas. 2001. 22: R35-66
12. Brouwer PA, Brand R, van den Akker-van Marle ME, Jacobs WC, Schenk B, van den Berg-Huijsmans AA. Percutaneous laser disc decompression versus conventional microdiscectomy in sciatica: A randomized controlled trial. Spine J. 2015. 15: 857-65
13. Byval’tsev VA, Panasenkov SY, Belykh EG, Ivanov NA, Tsyganov PY, Nikiforov SB. Nanostructure changes in the intervertebral discs after experimental laser irradiation. Bull Exp Biol Med. 2015. 158: 504-7
14. Byvaltsev V, Belykh E, Panasenkov S, Ivanov N, Tsyganov P, Sorokovikov V. Nanostructural changes of intervertebral disc after diode laser ablation. World Neurosurg. 2012. 77: 6-7
15. Calisto A, Dorfmuller G, Fohlen M, Bulteau C, Conti A, Delalande O. Endoscopic disconnection of hypothalamic hamartomas: Safety and feasibility of robot-assisted, thulium laser-based procedures. J Neurosurg Pediatr. 2014. 14: 563-72
16. Carpentier A, Chauvet D, Reina V, Beccaria K, Leclerq D, McNichols RJ. MR-guided laser-induced thermal therapy (LITT) for recurrent glioblastomas. Lasers Surg Med. 2012. 44: 361-8
17. Carpentier A, McNichols RJ, Stafford RJ, Guichard JP, Reizine D, Delaloge S. Laser thermal therapy: Real-time MRI-guided and computer-controlled procedures for metastatic brain tumors. Lasers Surg Med. 2011. 43: 943-50
18. Carpentier A, McNichols RJ, Stafford RJ, Itzcovitz J, Guichard JP, Reizine D. Real-time magnetic resonance-guided laser thermal therapy for focal metastatic brain tumors. Neurosurgery. 2008. 63: ONS21-
19. Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: Part two-cellular signaling, cell metabolism and modes of cell death. Photodiagnosis Photodyn Ther. 2005. 2: 1-23
20. Chao ST, Ahluwalia MS, Barnett GH, Stevens GH, Murphy ES, Stockham AL. Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int J Radiat Oncol Biol Phys. 2013. 87: 449-57
21. Choi JU, Kim DS, Huh R. Endoscopic approach to arachnoid cyst. Childs Nerv Syst. 1999. 15: 285-91
22. Choy DS. Percutaneous laser disc decompression (PLDD) update: Focus on device and procedure advances. J Clin Laser Med Surg. 1993. 11: 181-3
23. Choy DS. Percutaneous laser disc decompression: A 17-year experience. Photomed Laser Surg. 2004. 22: 407-10
24. Choy DS, Altman P, Trokel SL. Efficiency of disc ablation with lasers of various wavelengths. J Clin Laser Med Surg. 1995. 13: 153-6
25. Choy DS, Case RB, Fielding W, Hughes J, Liebler W, Ascher P. Percutaneous laser nucleolysis of lumbar disks. N Engl J Med. 1987. 317: 771-2
26. Crandall CG, Meyer DM, Davis SL, Dellaria SM. Palmar skin blood flow and temperature responses throughout endoscopic sympathectomy. Anesth Analg. 2005. 100: 277-83
27. Curry DJ, Gowda A, McNichols RJ, Wilfong AA. MR-guided stereotactic laser ablation of epileptogenic foci in children. Epilepsy Behav. 2012. 24: 408-14
28. Davis JK. Early experience with laser disc decompression. A percutaneous method. J Florida Med Assoc. 1992. 79: 37-9
29. Dippel EJ, Makam P, Kovach R, George JC, Patlola R, Metzger DC. Randomized controlled study of excimer laser atherectomy for treatment of femoropopliteal in-stent restenosis: Initial results from the EXCITE ISR trial (EXCImer Laser Randomized Controlled Study for Treatment of FemoropopliTEal In-Stent Restenosis). JACC Cardiovasc Intervent. 2015. 8: 92-101
30. Dunn AK, Bolay H, Moskowitz MA, Boas DA. Dynamic imaging of cerebral blood flow using laser speckle. J Cereb Blood Flow Metab. 2001. 21: 195-201
31. Eggert HR, Blazek V. Optical properties of human brain tissue, meninges, and brain tumors in the spectral range of 200 to 900 nm. Neurosurgery. 1987. 21: 459-64
32. El-Khatib M, Tepe C, Senger B, Dibue-Adjei M, Riemenschneider MJ, Stummer W. Aminolevulinic acid-mediated photodynamic therapy of human meningioma: An in vitro study on primary cell lines. Int J Mol Sci. 2015. 16: 9936-48
33. Elder JB, Chiocca EA. Editorial: Glioblastoma multiforme and laser interstitial thermal therapy. J Neurosurg. 2013. 118: 1199-
34. Eljamel MS. Brain photodiagnosis (PD), fluorescence guided resection (FGR) and photodynamic therapy (PDT): Past, present and future. Photodiagnosis Photodyn Ther. 2008. 5: 29-35
35. Eljamel MS, Goodman C, Moseley H. ALA and Photofrin fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: A single centre Phase III randomised controlled trial. Lasers Med Sci. 2008. 23: 361-7
36. Epstein NE. Should anyone perform percutaneous endoscopic laser diskectomy and percutaneous lumbar disc decompressions?. Surg Neurol Int. 2016. 7: S1080-4
37. Eschbacher J, Martirosyan NL, Nakaji P, Sanai N, Preul MC, Smith KA. In vivo intraoperative confocal microscopy for real-time histopathological imaging of brain tumors. J Neurosurg. 2012. 116: 854-60
38. Esquenazi Y, Kalamangalam GP, Slater JD, Knowlton RC, Friedman E, Morris SA. Stereotactic laser ablation of epileptogenic periventricular nodular heterotopia. Epilepsy Res. 2014. 108: 547-54
39. Eun HC. Evaluation of skin blood flow by laser Doppler flowmetry. Clin Dermatol. 1995. 13: 337-47
40. Fabiano AJ, Alberico RA. Laser-interstitial thermal therapy for refractory cerebral edema from post-radiosurgery metastasis. World Neurosurg. 2014. 81: 652.e651-4
41. Fabiano AJ, Qiu J. Delayed failure of laser-induced interstitial thermotherapy for postradiosurgery brain metastases. World Neurosurg. 2014. 82: e559-63
42. Fenton KE, Martirosyan NL, Abdelwahab MG, Coons SW, Preul MC, Scheck AC. In vivo visualization of GL261-luc2 mouse glioma cells by use of Alexa Fluor-labeled TRP-2 antibodies. Neurosurg Focus. 2014. 36: E12-
43. Foersch S, Heimann A, Ayyad A, Spoden GA, Florin L, Mpoukouvalas K. Confocal laser endomicroscopy for diagnosis and histomorphologic imaging of brain tumors in vivo. PloS One. 2012. 7: e41760-
44. Forer B, Vasileyev T, Gil Z, Brosh T, Kariv N, Katzir A. CO(2) laser fascia to dura soldering for pig dural defect reconstruction. Skull Base. 2007. 17: 17-23
45. Foyt D, Johnson JP, Kirsch AJ, Bruce JN, Wazen JJ. Dural closure with laser tissue welding. Otolaryngol Head Neck Surg. 1996. 115: 513-8
46. Gangi A, Basile A, Buy X, Alizadeh H, Sauer B, Bierry G. Radiofrequency and laser ablation of spinal lesions. Semin Ultrasound CT MR. 2005. 26: 89-97
47. Gangi A, Dietemann JL, Ide C, Brunner P, Klinkert A, Warter JM. Percutaneous laser disk decompression under CT and fluoroscopic guidance: Indications, technique, and clinical experience. Radiographics. 1996. 16: 89-96
48. Georges JF, Martirosyan NL, Eschbacher J, Nichols J, Tissot M, Preul MC. Sulforhodamine 101 selectively labels human astrocytoma cells in an animal model of glioblastoma. J Clin Neurosci. 2014. 21: 846-51
49. Gigo-Benato D, Geuna S, de Castro Rodrigues A, Tos P, Fornaro M, Boux E. Low-power laser biostimulation enhances nerve repair after end-to-side neurorrhaphy: A double-blind randomized study in the rat median nerve model. Lasers Med Sci. 2004. 19: 57-65
50. Gigo-Benato D, Geuna S, Rochkind S. Phototherapy for enhancing peripheral nerve repair: A review of the literature. Muscle Nerve. 2005. 31: 694-701
51. Gilligan DM, Dan D. Excimer laser for pacemaker and defibrillator lead extraction: Techniques and clinical results. Lasers Med Sci. 2001. 16: 113-21
52. Goetz M, Deris I, Vieth M, Murr E, Hoffman A, Delaney P. Near-infrared confocal imaging during mini-laparoscopy: A novel rigid endomicroscope with increased imaging plane depth. J Hepatol. 2010. 53: 84-90
53. Gonzalez-Martinez J, Vadera S, Mullin J, Enatsu R, Alexopoulos AV, Patwardhan R. Robot-assisted stereotactic laser ablation in medically intractable epilepsy: Operative technique. Neurosurgery. 2014. 10: 167-
54. Gravvanis AI, Lavdas AA, Papalois A, Tsoutsos DA, Matsas R. The beneficial effect of genetically engineered Schwann cells with enhanced motility in peripheral nerve regeneration: Review. Acta Neurochir Suppl. 2007. 100: 51-6
55. Greco M, Guida G, Perlino E, Marra E, Quagliariello E. Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem Biophys Res Comm. 1989. 163: 1428-34
56. Hacke W, Schellinger PD, Albers GW, Bornstein NM, Dahlof BL, Fulton R. Transcranial laser therapy in acute stroke treatment: Results of neurothera effectiveness and safety trial 3, a phase III clinical end point device trial. Stroke. 2014. 45: 3187-93
57. Hadley MN, Martin NA, Spetzler RF, Sonntag VK, Johnson PC. Comparative transoral dural closure techniques: A canine model. Neurosurgery. 1988. 22: 392-7
58. Hashmi JT, Huang YY, Osmani BZ, Sharma SK, Naeser MA, Hamblin MR. Role of low-level laser therapy in neurorehabilitation. PM R. 2010. 2: S292-305
59. Hawasli AH, Kim AH, Dunn GP, Tran DD, Leuthardt EC. Stereotactic laser ablation of high-grade gliomas. Neurosurg Focus. 2014. 37: E1-
60. Hellinger J. Complications of non-endoscopic percutaneous laser disc decompression and nucleotomy with the neodymium: YAG laser 1064 nm. Photomed Laser Surg. 2004. 22: 418-22
61. Hendrikse J, van der Zwan A, Ramos LM, Tulleken CA, van der Grond J. Hemodynamic compensation via an excimer laser-assisted, high-flow bypass before and after therapeutic occlusion of the internal carotid artery. Neurosurgery. 2003. 53: 858-
62. Hu D, Jiang Y, Belykh E, Gong Y, Preul MC, Hannaford B. Toward real-time tumor margin identification in image-guided robotic brain tumor resection. 2017. p.
63. Jabbour JM, Saldua MA, Bixler JN, Maitland KC. Confocal endomicroscopy: Instrumentation and medical applications. Ann Biomed Eng. 2012. 40: 378-97
64. Jarvik JG, Deyo RA. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med. 2002. 137: 586-97
65. Jenkins SD, Sepka RS, Barwick WJ, Serafin D, Klitzman B. Routine clinical use of laser Doppler flowmeter to monitor free tissue transfer: Preliminary results. J Reconstr Microsurg. 1987. 3: 281-3
66. Jermyn M, Mok K, Mercier J, Desroches J, Pichette J, Saint-Arnaud K. Intraoperative brain cancer detection with Raman spectroscopy in humans. Sci Transl Med. 2015. 7: 274ra219-
67. Jo DH, Kim ED, Oh HJ. The comparison of the result of epiduroscopic laser neural decompression between FBSS or not. Korean J Pain. 2014. 27: 63-7
68. Jo DH, Yang HJ. The survey of the patient received the epiduroscopic laser neural decompression. Korean J Pain. 2013. 26: 27-31
69. Jo DH, Yang HJ, Kim JJ. Approach for epiduroscopic laser neural decompression in case of the sacral canal stenosis. Korean J Pain. 2013. 26: 392-5
70. Johansson A, Faber F, Kniebuhler G, Stepp H, Sroka R, Egensperger R. Protoporphyrin IX fluorescence and photobleaching during interstitial photodynamic therapy of malignant gliomas for early treatment prognosis. Lasers Surg Med. 2013. 45: 225-34
71. Kalani MY, Zabramski JM, Hu YC, Spetzler RF. Extracranial-intracranial bypass and vessel occlusion for the treatment of unclippable giant middle cerebral artery aneurysms. Neurosurgery. 2013. 72: 428-
72. Kiesslich R, Burg J, Vieth M, Gnaendiger J, Enders M, Delaney P. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004. 127: 706-13
73. Kingwell BA, Formosa M, Muhlmann M, Bradley SJ, McConell GK. Type 2 diabetic individuals have impaired leg blood flow responses to exercise: Role of endothelium-dependent vasodilation. Diabetes Care. 2003. 26: 899-904
74. Klein KU, Fukui K, Schramm P, Stadie A, Fischer G, Werner C. Human cerebral microcirculation and oxygen saturation during propofol-induced reduction of bispectral index. Br J Anaesth. 2011. 107: 735-41
75. Knappe V, Frank F, Rohde E. Principles of lasers and biophotonic effects. Photomed Laser Surg. 2004. 22: 411-7
76. Koljenovic S, Schut TB, Vincent A, Kros JM, Puppels GJ. Detection of meningioma in dura mater by Raman spectroscopy. Anal Chem. 2005. 77: 7958-65
77. Komel’kova LV, Vitreshchak TV, Zhirnova IG, Poleshchuk VV, Stvolinskii SL, Mikhailov VV. [Biochemical and immunological induces of the blood in Parkinson's disease and their correction with the help of laser therapy]. Patologicheskaia fiziologiia i eksperimental’nia terapiia. 2004. p. 15-8
78. Kostron H, Fiegele T, Akatuna E. Combination of FOSCAN mediated fluorescence guided resection and photodynamic treatment as new therapeutic concept for malignant brain tumors. Med Laser Appl. 2006. 21: 285-90
79. Krafft C, Sobottka SB, Schackert G, Salzer R. Near infrared Raman spectroscopic mapping of native brain tissue and intracranial tumors. The Analyst. 2005. 130: 1070-7
80. Krishnamurthy S, Powers SK. Lasers in neurosurgery. Lasers Surg Med. 1994. 15: 126-67
81. Laemmel E, Genet M, Le Goualher G, Perchant A, Le Gargasson JF, Vicaut E. Fibered confocal fluorescence microscopy (Cell-viZio) facilitates extended imaging in the field of microcirculation. A comparison with intravital microscopy. J Vasc Res. 2004. 41: 400-11
82. Lampl Y. Laser treatment for stroke. Exp Rev Neurother. 2007. 7: 961-5
83. Lampl Y, Zivin JA, Fisher M, Lew R, Welin L, Dahlof B. Infrared laser therapy for ischemic stroke: A new treatment strategy: Results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1). Stroke. 2007. 38: 1843-9
84. Lapchak PA, De Taboada L. Transcranial near infrared laser treatment (NILT) increases cortical adenosine-5’-triphosphate (ATP) content following embolic strokes in rabbits. Brain Res. 2010. 1306: 100-5
85. Lauritzen M, Fabricius M. Real time laser-Doppler perfusion imaging of cortical spreading depression in rat neocortex. Neuroreport. 1995. 6: 1271-3
86. Lohr NL, Keszler A, Pratt P, Bienengraber M, Warltier DC, Hogg N. Enhancement of nitric oxide release from nitrosyl hemoglobin and nitrosyl myoglobin by red/near infrared radiation: Potential role in cardioprotection. J Mol Cell Cardiol. 2009. 47: 256-63
87. Longo L.editorsTerapia Laser. Firenze: USES; 1986. p.
88. Madduri S, Gander B. Schwann cell delivery of neurotrophic factors for peripheral nerve regeneration. J Peripher Nerv Syst. 2010. 15: 93-103
89. Martirosyan NL, Cavalcanti DD, Eschbacher JM, Delaney PM, Scheck AC, Abdelwahab MG. Use of in vivo near-infrared laser confocal endomicroscopy with indocyanine green to detect the boundary of infiltrative tumor. J Neurosurg. 2011. 115: 1131-8
90. Martirosyan NL, Eschbacher JM, Kalani MY, Turner JD, Belykh E, Spetzler RF. Prospective evaluation of the utility of intraoperative confocal laser endomicroscopy in patients with brain neoplasms using fluorescein sodium: Experience with 74 cases. Neurosurg Focus. 2016. 40: E11-
91. Martirosyan NL, Georges J, Eschbacher JM, Cavalcanti DD, Elhadi AM, Abdelwahab MG. Potential application of a handheld confocal endomicroscope imaging system using a variety of fluorophores in experimental gliomas and normal brain. Neurosurg Focus. 2014. 36: E16-
92. McNichols RJ, Gowda A, Kangasniemi M, Bankson JA, Price RE, Hazle JD. MR thermometry-based feedback control of laser interstitial thermal therapy at 980 nm. Lasers Surg Med. 2004. 34: 48-55
93. Menchetti PP, Canero G, Bini W. Percutaneous laser discectomy: Experience and long term follow-up. Acta Neurochir Suppl. 2011. 108: 117-121
94. Meng C, He Z, Xing D. Low-level laser therapy rescues dendrite atrophy via upregulating BDNF expression: Implications for Alzheimer's disease. J Neurosci. 2013. 33: 13505-17
95. Mester E, Spiry T, Szende B, Tota JG. Effect of laser rays on wound healing. Am J Surg. 1971. 122: 532-5
96. Mester E, Szende B, Gartner P. [The effect of laser beams on the growth of hair in mice]. [German] Radiobiol Radiother. 1968. 9: 621-6
97. Mester E, Szende B, Spiry T, Scher A. Stimulation of wound healing by laser rays. Acta Chir Acad Sci Hung. 1972. 13: 315-24
98. Moges H, Vasconcelos OM, Campbell WW, Borke RC, McCoy JA, Kaczmarczyk L. Light therapy and supplementary Riboflavin in the SOD1 transgenic mouse model of familial amyotrophic lateral sclerosis (FALS). Lasers Surg Med. 2009. 41: 52-9
99. Mohammed IF, Al-Mustawfi N, Kaka LN. Promotion of regenerative processes in injured peripheral nerve induced by low-level laser therapy. Photomed Laser Surg. 2007. 25: 107-11
100. Moreira MS, Velasco IT, Ferreira LS, Ariga SK, Barbeiro DF, Meneguzzo DT. Effect of phototherapy with low intensity laser on local and systemic immunomodulation following focal brain damage in rat. J Photochem Photobiol Biol. 2009. 97: 145-51
101. Muller PJ, Wilson BC. Photodynamic therapy of brain tumors-a work in progress. Lasers Surg Med. 2006. 38: 384-9
102. Muragaki Y, Akimoto J, Maruyama T, Iseki H, Ikuta S, Nitta M. Phase II clinical study on intraoperative photodynamic therapy with talaporfin sodium and semiconductor laser in patients with malignant brain tumors. J Neurosurg. 2013. 119: 845-52
103. Nejat F, El Khashab M, Rutka JT. Initial management of childhood brain tumors: Neurosurgical considerations. J Child Neurol. 2008. 23: 1136-48
104. Norred SE, Johnson JA. Magnetic resonance-guided laser induced thermal therapy for glioblastoma multiforme: A review. BioMed Res Int 2014. 2014. p. 761312-
105. Oron A, Oron U, Chen J, Eilam A, Zhang C, Sadeh M. Low-level laser therapy applied transcranially to rats after induction of stroke significantly reduces long-term neurological deficits. Stroke. 2006. 37: 2620-4
106. Oron A, Oron U, Streeter J, de Taboada L, Alexandrovich A, Trembovler V. low-level laser therapy applied transcranially to mice following traumatic brain injury significantly reduces long-term neurological deficits. J Neurotrauma. 2007. 24: 651-6
107. Palmer JC, Baig S, Kehoe PG, Love S. Endothelin-converting enzyme-2 is increased in Alzheimer's disease and up-regulated by Abeta. Am J Pathol. 2009. 175: 262-70
108. Patel NV, Jethwa PR, Shetty A, Danish SF. Does the real-time thermal damage estimate allow for estimation of tumor control after MRI-guided laser-induced thermal therapy? Initial experience with recurrent intracranial ependymomas. J Neurosurg Pediatr. 2015. 15: 363-71
109. Pellaton C, Kubli S, Feihl F, Waeber B. Blunted vasodilatory responses in the cutaneous microcirculation of cigarette smokers. Am Heart J. 2002. 144: 269-74
110. Powers SK. Fenestration of intraventricular cysts using a flexible, steerable endoscope and the argon laser. Neurosurgery. 1986. 18: 637-41
111. Powers SK, Brown JT. Light dosimetry in brain tissue: An in vivo model applicable to photodynamic therapy. Lasers Surg Med. 1986. 6: 318-22
112. Quirk BJ, Brandal G, Donlon S, Vera JC, Mang TS, Foy AB. Photodynamic therapy (PDT) for malignant brain tumors - where do we stand?. Photodiagn Photodynamic Ther. 2015. 12: 530-44
113. Rahmathulla G, Recinos PF, Valerio JE, Chao S, Barnett GH. Laser interstitial thermal therapy for focal cerebral radiation necrosis: A case report and literature review. Stereotact Funct Neurosurg. 2012. 90: 192-200
114. Rao MS, Hargreaves EL, Khan AJ, Haffty BG, Danish SF. Magnetic resonance-guided laser ablation improves local control for postradiosurgery recurrence and/or radiation necrosis. Neurosurgery. 2014. 74: 658-67
115. Rochkind S, Leider-Trejo L, Nissan M, Shamir MH, Kharenko O, Alon M. Efficacy of 780-nm laser phototherapy on peripheral nerve regeneration after neurotube reconstruction procedure (double-blind randomized study). Photomed Laser Surg. 2007. 25: 137-43
116. Rosenthal MA, Kavar B, Hill JS, Morgan DJ, Nation RL, Stylli SS. Phase I and pharmacokinetic study of photodynamic therapy for high-grade gliomas using a novel boronated porphyrin. J Clin Oncol. 2001. 19: 519-24
117. Rosomoff HL, Carroll F. Reaction of neoplasm and brain to laser. Arch Neurol. 1966. 14: 143-8
118. Ruth B. Blood flow determination by the laser speckle method. Int J Microcirc Clin Exp. 1990. 9: 21-45
119. Ryan RW, Spetzler RF, Preul MC. Aura of technology and the cutting edge: A history of lasers in neurosurgery. Neurosurg Focus. 2009. 27: E6-
120. Ryan RW, Wolf T, Spetzler RF, Coons SW, Fink Y, Preul MC. Application of a flexible CO(2) laser fiber for neurosurgery: Laser-tissue interactions. J Neurosurg. 2010. 112: 434-43
121. Salcman M, Samaras GM. Hyperthermia for brain tumors: Biophysical rationale. Neurosurgery. 1981. 9: 327-35
122. Sanai N, Snyder LA, Honea NJ, Coons SW, Eschbacher JM, Smith KA. Intraoperative confocal microscopy in the visualization of 5-aminolevulinic acid fluorescence in low-grade gliomas. J Neurosurg. 2011. 115: 740-8
123. Sankar T, Delaney PM, Ryan RW, Eschbacher J, Abdelwahab M, Nakaji P. Miniaturized handheld confocal microscopy for neurosurgery: Results in an experimental glioblastoma model. Neurosurgery. 2010. 66: 410-
124. Schenk B, Brouwer PA, Peul WC, van Buchem MA. Percutaneous laser disk decompression: A review of the literature. AJNR Am J Neuroradiol. 2006. 27: 232-5
125. Schlosser HG, Suess O, Vajkoczy P, van Landeghem FK, Zeitz M, Bojarski C. Confocal neurolasermicroscopy in human brain - perspectives for neurosurgery on a cellular level (including additional comments to this article). Cent Eur Neurosurg. 2010. 71: 13-9
126. Schmidt MH, Bajic DM, Reichert KW, Martin TS, Meyer GA, Whelan HT. Light-emitting diodes as a light source for intraoperative photodynamic therapy. Neurosurgery. 1996. 38: 552-
127. Schmidt MH, Meyer GA, Reichert KW, Cheng J, Krouwer HG, Ozker K. Evaluation of photodynamic therapy near functional brain tissue in patients with recurrent brain tumors. J Neurooncol. 2004. 67: 201-7
128. Seibel EJ, Brown CM, Dominitz JA, Kimmey MB. Scanning single fiber endoscopy: A new platform technology for integrated laser imaging, diagnosis, and future therapies. Gastrointest Endosc Clin N Am. 2008. 18: 467-78, viii
129. Semwogerere D, Weeks ER.editorsEncyclopedia of Biomaterials and Biomedical Engineering. Atlanta: Taylor & Francis; 2005. p.
130. Singh V, Manchikanti L, Calodney AK, Staats PS, Falco FJ, Caraway DL. Percutaneous lumbar laser disc decompression: An update of current evidence. Pain Phys. 2013. 16: SE229-60
131. Sloan AE, Ahluwalia MS, Valerio-Pascua J, Manjila S, Torchia MG, Jones SE. Results of the NeuroBlate System first-in-humans Phase I clinical trial for recurrent glioblastoma: Clinical article. J Neurosurg. 2013. 118: 1202-19
132. Stecher D, Bronkers G, Noest JO, Tulleken CA, Hoefer IE, van Herwerden LA. Evaluation of a novel laser-assisted coronary anastomotic connector - the Trinity Clip - in a porcine off-pump bypass model. J Vis Exp. 2014. p. e52127-
133. Stecher D, de Boer B, Tulleken CA, Pasterkamp G, van Herwerden LA, Buijsrogge MP. A new nonocclusive laser-assisted coronary anastomotic connector in a rabbit model. J Thorac Cardiovasc Surg. 2013. 145: 1124-9
134. Streefkerk HJ, Wolfs JF, Sorteberg W, Sorteberg AG, Tulleken CA. The ELANA technique: Constructing a high flow bypass using a non-occlusive anastomosis on the ICA and a conventional anastomosis on the SCA in the treatment of a fusiform giant basilar trunk aneurysm. Acta Neurochir. 2004. 146: 1009-19
135. Stupak VV, Pendjurin IV, Zaidman AM, Strutz SG. Experimental and clinical backgrounds for applying Photosense in photodynamic therapy in neurooncological patients. [article in Russian]. Lazernaya Meditsina. 2007. 11: 40-6
136. Stylli SS, Kaye AH, MacGregor L, Howes M, Rajendra P. Photodynamic therapy of high grade glioma - long term survival. J Clin Neurosci. 2005. 12: 389-8
137. Sundt TM, Piepgras DG, Marsh WR, Fode NC. Saphenous vein bypass grafts for giant aneurysms and intracranial occlusive disease. J Neurosurg. 1986. 65: 439-50
138. Svaasand LO. Photodynamic and photohyperthermic response of malignant tumors. Med Phys. 1985. 12: 455-61
139. Svaasand LO, Ellingsen R. Optical properties of human brain. Photochem Photobiol. 1983. 38: 293-9
140. Takizawa T. The carbon dioxide laser surgical unit as an instrument for surgery of brain tumours--its advantages and disadvantages. Neurosurg Rev. 1984. 7: 135-44
141. Tetard MC, Vermandel M, Mordon S, Lejeune JP, Reyns N. Experimental use of photodynamic therapy in high grade gliomas: A review focused on 5-aminolevulinic acid. Photodiagn Photodynamic Ther. 2014. 11: 319-30
142. Torres-Reveron J, Tomasiewicz HC, Shetty A, Amankulor NM, Chiang VL. Stereotactic laser induced thermotherapy (LITT): A novel treatment for brain lesions regrowing after radiosurgery. J Neurooncol. 2013. 113: 495-503
143. Trimmer PA, Schwartz KM, Borland MK, De Taboada L, Streeter J, Oron U. Reduced axonal transport in Parkinson's disease cybrid neurites is restored by light therapy. Mol Neurodegener. 2009. 4: 26-
144. Uozumi Y, Nawashiro H, Sato S, Kawauchi S, Shima K, Kikuchi M. Targeted increase in cerebral blood flow by transcranial near-infrared laser irradiation. Lasers Surg Med. 2010. 42: 566-76
145. van Beijnum J, Hanlo PW, Fischer K, Majidpour MM, Kortekaas MF, Verdaasdonk RM. Laser-assisted endoscopic third ventriculostomy: Long-term results in a series of 202 patients. Neurosurgery. 2008. 62: 437-
146. Van Beijnum J, Hanlo PW, Han KS, Ludo Van der Pol W, Verdaasdonk RM, Van Nieuwenhuizen O. Navigated laser-assisted endoscopic fenestration of a suprasellar arachnoid cyst in a 2-year-old child with bobble-head doll syndrome. Case report. J Neurosurg. 2006. 104: 348-51
147. van der Zwan A, Tulleken CA, Hillen B. Flow quantification of the non-occlusive excimer laser-assisted EC-IC bypass. Acta Neurochir. 2001. 143: 647-54
148. van Doormaal TP, van der Zwan A, Aboud E, Berkelbach van der Sprenkel JW, Tulleken CA, Krisht AF. The sutureless excimer laser assisted non-occlusive anastomosis (SELANA); a feasibility study in a pressurized cadaver model. Acta Neurochir. 2010. 152: 1603-
149. Vandertop WP, Verdaasdonk RM, van Swol CF. Laser-assisted neuroendoscopy using a neodymium-yttrium aluminum garnet or diode contact laser with pretreated fiber tips. J Neurosurg. 1998. 88: 82-92
150. Wang CZ, Chen YJ, Wang YH, Yeh ML, Huang MH, Ho ML. Low-level laser irradiation improves functional recovery and nerve regeneration in sciatic nerve crush rat injury model. PloS One. 2014. 9: e103348-
151. Wang Z, Zhong H, Yang Z, Zhao F, Wang B, Qu P. Exogenous bFGF or TGFbeta1 accelerates healing of reconstructed dura by CO2 laser soldering in minipigs. Lasers Med Sci. 2014. 29: 1165-71
152. Wardell K, Blomstedt P, Richter J, Antonsson J, Eriksson O, Zsigmond P. Intracerebral microvascular measurements during deep brain stimulation implantation using laser Doppler perfusion monitoring. Stereotact Funct Neurosurg. 2007. 85: 279-86
153. Wong-Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: Role of cytochrome c oxidase. J Biol Chem. 2005. 280: 4761-71
154. Wu Q, Xuan W, Ando T, Xu T, Huang L, Huang YY. Low-level laser therapy for closed-head traumatic brain injury in mice: Effect of different wavelengths. Lasers Surg Med. 2012. 44: 218-26
155. Yaoeda K, Shirakashi M, Funaki S, Funaki H, Nakatsue T, Fukushima A. Measurement of microcirculation in optic nerve head by laser speckle flowgraphy in normal volunteers. Am J Ophthalmol. 2000. 130: 606-10
156. Zehri AH, Ramey W, Georges JF, Mooney MA, Martirosyan NL, Preul MC. Neurosurgical confocal endomicroscopy: A review of contrast agents, confocal systems, and future imaging modalities. Surg Neurol Int. 2014. 5: 60-
157. Zivin JA, Albers GW, Bornstein N, Chippendale T, Dahlof B, Devlin T. Effectiveness and safety of transcranial laser therapy for acute ischemic stroke. Stroke. 2009. 40: 1359-64