- Department of Anaesthesiology, Instituto de Neurocirugía Asenjo, Providencia, Santiago, Chile
- Neurosurgery Service, Hospital Regional Libertador Bernardo O’Higgins, Rancagua, Chile
- Chief of Neuroradiology Service, Instituto de Neurocirugía Asenjo, Providencia, Santiago, Chile
- Neurology Service, Hospital Regional Libertador Bernardo O’Higgins, Rancagua, Chile
- Department of Neurological Sciences, Universidad de Chile, Santiago, Chile
- Neurosurgery Service, Instituto de Neurocirugía Asenjo, Providencia, Santiago, Chile
- Chief of Neurosurgery Service, Hospital Regional Libertador Bernardo O’Higgins, Rancagua, and Department of Neurosurgery, Universidad de Chile, Santiago, Chile
- Chief of Cerebrovascular and Skull Base Surgery, Instituto de Neurocirugía Asenjo, Providencia, Santiago, Chile
Correspondence Address:
Rodrigo Zapata
Neurosurgery Service, Hospital Regional Libertador Bernardo O'Higgins, Rancagua, Chile
DOI:10.4103/sni.sni_266_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Geisi Saito, Rodrigo Zapata, Rodrigo Rivera, Héctor Zambrano, David Rojas, Hernán Acevedo, Franco Ravera, John Mosquera, Juan E. Vásquez, Jorge Mura. Long-chain omega-3 fatty acids in aneurysmal subarachnoid hemorrhage: A randomized pilot trial of pharmaconutrition. 27-Dec-2017;8:304
How to cite this URL: Geisi Saito, Rodrigo Zapata, Rodrigo Rivera, Héctor Zambrano, David Rojas, Hernán Acevedo, Franco Ravera, John Mosquera, Juan E. Vásquez, Jorge Mura. Long-chain omega-3 fatty acids in aneurysmal subarachnoid hemorrhage: A randomized pilot trial of pharmaconutrition. 27-Dec-2017;8:304. Available from: http://surgicalneurologyint.com/surgicalint-articles/long%e2%80%91chain-omega%e2%80%913-fatty-acids-in-aneurysmal-subarachnoid-hemorrhage-a-randomized-pilot-trial-of-pharmaconutrition/
Abstract
Background:Functional recovery after aneurysmal subarachnoid hemorrhage (SAH) remains a significant problem. We tested a novel therapeutic approach with long-chain omega-3 polyunsaturated fatty acids (n-3 PUFAs) to assess the safety and feasibility of an effectiveness trial.
Methods:We conducted a multicentre, parallel, randomized, open-label pilot trial. Patients admitted within 72 hours after SAH with modified Fisher scale scores of 3 or 4 who were selected for scheduled aneurysm clipping were allocated to receive either n-3 PUFA treatment (parenteral perioperative: 5 days; oral: 8 weeks) plus usual care or usual care alone. Exploratory outcome measures included major postoperative intracranial bleeding complications (PIBCs), cerebral infarction caused by delayed cerebral ischemia, shunt-dependent hydrocephalus, and consent rate. The computed tomography evaluator was blinded to the group assignment.
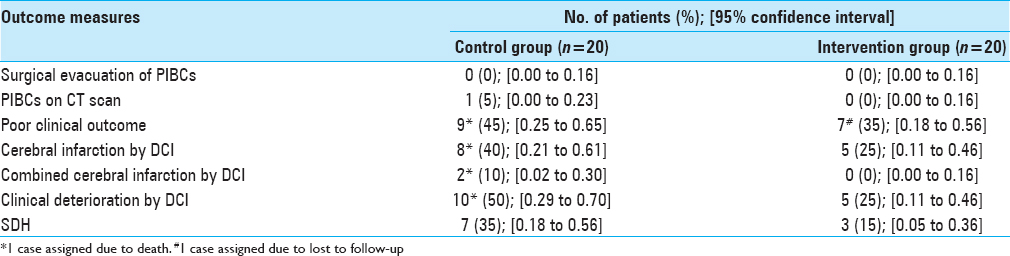
Results:Forty-one patients were randomized, but one patient had to be excluded after allocation. Twenty patients remained for intention to treat analysis in each trial arm. No PIBs (95% confidence interval [CI]: 0.00 to 0.16) or other unexpected harm were observed in the intervention group (IG). No patient suspended the intervention due to side effects. There was a trend towards improvements in all benefit-related outcomes in the IG. The overall consent rate was 0.91 (95% CI: 0.78 to 0.96), and there was no consent withdrawal.
Conclusions:Although the balance between the benefit and harm of the intervention appears highly favourable, further testing on SAH patients is required. We recommend proceeding with amendments in a dose-finding trial to determine the optimal duration of parenteral treatment.
Keywords: Omega-3 fatty acids, pharmaconutrition, pharmaconutrients, randomized pilot trial, subarachnoid hemorrhage
INTRODUCTION
Functional recovery after aneurysmal subarachnoid hemorrhage (SAH) remains a significant problem.[
For over 30 years, the long-chain omega-3 polyunsaturated fatty acids (n-3 PUFAs) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have been linked to major health benefits.[
Although fish oil (FO) in different clinical formulations has a high safety profile, no clinical trial of EPA plus DHA in SAH had previously been reported.[
MATERIALS AND METHODS
The study protocol was approved by a central medical ethics committee at each participating centre and by the government regulatory agency, “Instituto de Salud Pública de Chile” (
Study design
This study was a pragmatic, multicentre, open-label, randomized pilot clinical trial of 2 parallel groups (allocation ratio of 1:1).[
Patients and participating centres
The study was conducted at two public health care centres in Chile between March 2013 and October 2016. The “Instituto de Neurocirugía Asenjo” (INCA [
Patients were 18–69 years of age with a modified Fisher grade 3 or 4 SAH (short axis of cisternal blood on computed tomography [CT] ≥4 mm) who were admitted to the emergency department during the first 72 hours after the initial bleeding episode. Eligible patients had a ruptured intracranial aneurysm of the anterior circulation demonstrated by computed tomographic angiography (CTA) or digital subtraction angiography (DSA). Patients were required to be scheduled for surgical clipping no earlier than 5 hours after CTA/DSA diagnosis. The patient's clinical condition at admission or following medical stabilization or external ventricular drainage (EVD) placement was required to be World Federation of Neurological Surgeons (WFNS) grades 1–4. Eligible patients could not have severe unstable acute or chronic systemic disturbances, nor could they have received antiplatelets, anticoagulants or valproic acid during the 3 weeks before SAH. A history of allergy to iodine contrast media, fish or eggs; cerebral sequelae visible on admission CT; and refusal of informed consent were additional exclusion criteria. Legal representatives of eligible patients were approached by a researcher or attending physician to provide informed consent. Patients had to be stable (hemodynamic: 70 < mean arterial pressure (MAP) <130 mm Hg; 90 < systolic blood pressure (SBP) <180 mm Hg and headache <6 in the visual analogue scale [VAS]) at the intensive or intermediate care unit (ICU or IMCU) before randomization.
Randomization
Patients were randomized to treatment groups using sequentially numbered opaque sealed envelopes (SNOSEs) according to the literature.[
Allocation concealment
The SNOSEs were placed and stapled within transparent plastic envelopes and filed in numerical order in a folder. This folder was stored in a safe with an access code and nursing surveillance to prevent any further manipulation.
Implementation
The determination to enrol and allocate patients to treatment groups was made by an attending neurosurgeon who had to confirm this decision with one of the principal investigators. The SNOSEs were opened according to specific instructions, and the procedure was witnessed by two professionals. The allocation card containing the transcribed data was kept attached to each individual case report form for future audit.
Intervention[ 30 ]
A bimodal regimen of pharmaconutrition with n-3 PUFAs and controlled dietary supplementation of n-6 PUFAs was implemented as follows:
Parenteral treatment
A single daily dose of 100 ml of the FO-based lipid emulsion (FOLE), Omegaven 10% (Fresenius-Kabi Germany), was administered intravenously for 5 consecutive days under nursing surveillance at the ICU/IMCU. The first dose of FOLE had to be fully administered before beginning anaesthesia for aneurysm surgery and was continued thereafter every 24 hours. The infusion was performed by an infusion pump at 25 ml/hour via a central or peripheral venous line. However, after recruiting 22 subjects, we amended the protocol to increase the infusion rate to the maximum of 0.5 ml/kg body weight (BW)/hour, thus shortening the infusion time. Patients with a BW less than 50 kg were required to receive an adjusted dose of 0.2 g FO/kg BW/day. Concurrent administration of FOLE with iodinated contrast media was avoided. Flushing the residual volume of FOLE (12 ml) in the infusion line at the end of the procedure was not part of the protocol. This nursing procedure should always be performed to ensure full administration of the selected dose. Detailed information regarding FOLE is published elsewhere.[
Oral treatment
Four high-concentration FO capsules, Omacor 1000 mg (Ferrer Chile/Spain - Pronova BioPharma Norway), each containing 460 mg of EPA and 380 mg of DHA as ethyl esters, were given daily beginning the day after the last dose of FOLE until the 60th day after SAH. The daily dose was fractionated twice a day and given orally with the main meals under nursing assistant surveillance at the ICU/IMCU or the neurosurgical ward. SAH patients in poor clinical condition and those with swallowing disturbances received the oil contained in the capsules by an emulsion via a nasogastric tube. Although flushing the nasogastric tube with 20 ml of water after use is a regular nursing procedure, it was not protocolized. Capsules were given to the patient by a relative during outpatient care.
Basal diet
The dietary provision of n-6 PUFAs (by regular diet or enteral formula) did not exceed 9 g daily, and no additional n-3 PUFAs were given during the hospital stay. Thus, the total n-6/n-3 PUFA supplementation ratio did not exceed 2.5/1. Clinical dieticians supervised compliance with these instructions. Patients and their relatives received written nutritional recommendations to be followed during outpatient care: fish and seafood consumption had to be avoided during the treatment. Thereafter, patients were required to begin eating two portions of fatty fish twice weekly until the third month after SAH.[
Potential drug interactions
Statins, acetylsalicylic acid, and selective inhibitors of non-steroidal anti-inflammatory drugs (NSAIDs) had to be avoided for 30, 60, and 90 days respectively, as they theoretically have the potential to modify the effects of the intervention.[
Supplemental information regarding the intervention
A parenteral perioperative regimen may significantly shorten the time to an effective treatment, and surgically treated SAH patients may receive further clinical benefits.[
Usual care
The standard of care of SAH patients during the study period was based on the Explicit Health Guarantee (GES 2007) statement published by the Chilean Ministry of Public Health (
Outcome measures
In addition to recently recommended clinical endpoints, we added exploratory outcome measures to test new hypotheses and rule out major bleeding complications associated with high morbimortality rates.[
Clinical and radiological assessment
Clinical safety monitoring was provided to all patients during the hospital stay by a dedicated investigator. Two experienced neurosurgeons/neurointensivists established the diagnosis of neurological deterioration attributed to DCI. A neurologist/neurosurgeon conducted the interview (in person or by telephone with the patient or their relative) to assess GOSE and determine the diagnosis of SDH at 90 days. A single experienced neuroradiologist evaluated and compared the admission and follow-up CT scans obtained at 24 to 48 hours postoperatively or when clinically indicated. An additional cerebral CT scan was always obtained approximately 5 to 6 weeks after SAH to establish the definitive diagnosis of cerebral infarction and to rule out a more insidious development of chronic hydrocephalus.[
Data lost
Unavailable data from patients lost to follow-up were exclusively assigned to the benefit-related outcomes, according to the following criteria: In case of death, patients were regarded as having cerebral infarction and clinical deterioration due to DCI and poor clinical outcome. In case of lost to follow-up at three months, patients were regarded as having poor clinical outcome when the last available score in the GCS was <11.[
Statistics
We choose a sample size of 50 subjects, as this number would provide clinically relevant information while maintaining ethical requirements.[
RESULTS
Early stopping[ 17 ]
Patient enrolment was stopped before reaching the intended recruitment of 50 subjects, as the planned interval for trial execution of 3 years was exceeded. The main rationale behind this decision (made by the principal investigators) was that an adverse event rate of interest equal to zero in the IG group would provide almost the same estimate for 20 as for 25 subjects.[
Flow of participants
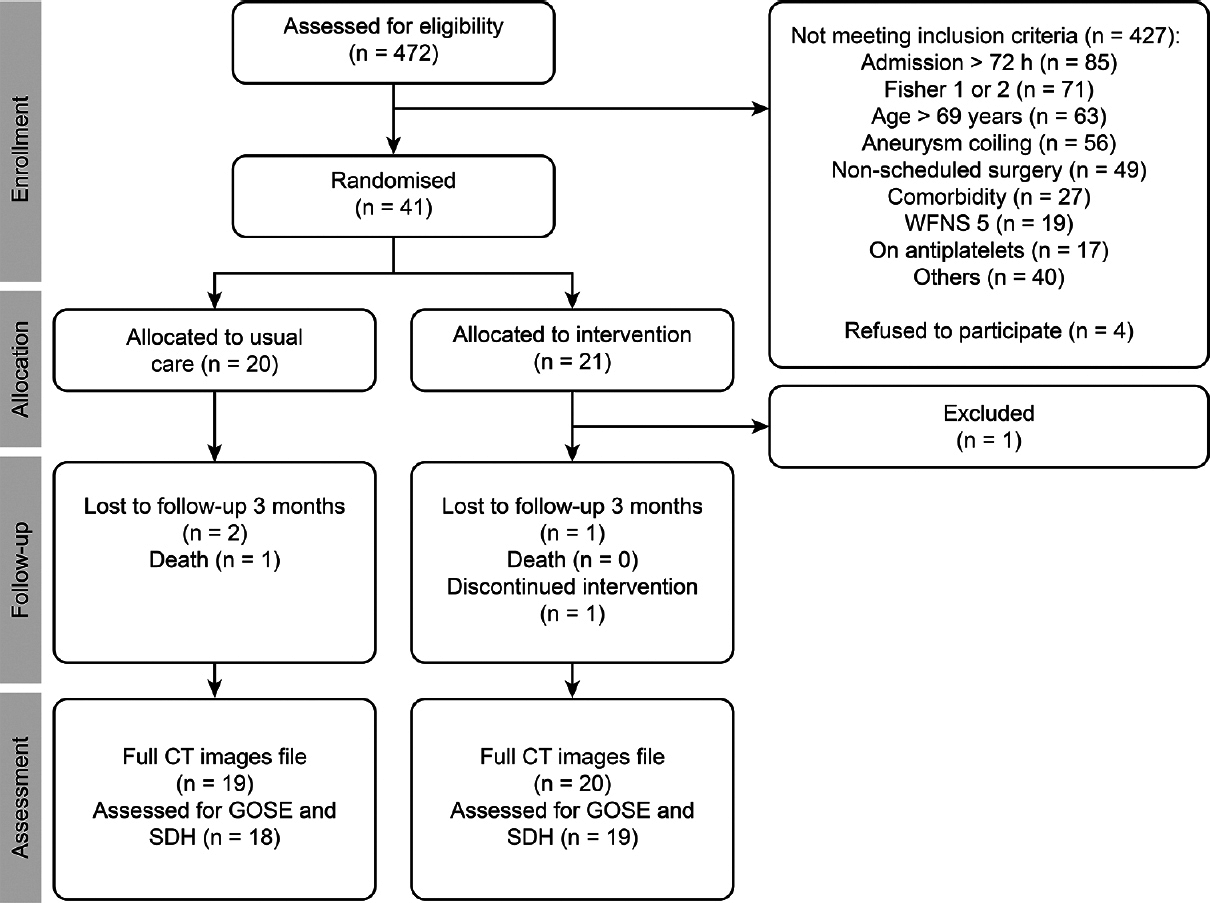
A flow diagram of the participants through each phase of the trial is depicted in
Demographic and clinical characteristics
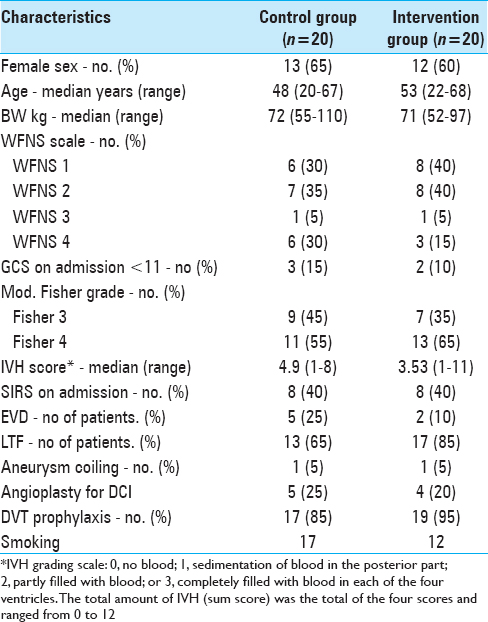
The demographic and clinical characteristics of both groups are shown in
Outcomes
Benefit- and harm-related outcomes are shown in
Protocol violations[ 9 ]
Protocol violations concerning admission criteria occurred in two cases (the aforementioned patient and another patient on antiplatelets and allocated to the CG). The therapeutic strategy was changed to endovascular treatment after allocation in two patients (one in each trial arm). The intervention was discontinued after one dose of FOLE in the endovascularly treated patient due to safety concerns, but this case was also included in the intention to treat analysis. Furthermore, the recruitment of one patient was not approved by the principal investigators, and one dose of FOLE was administered at a greater infusion rate than the maximum recommended.
DISCUSSION
The intervention did not increase the occurrence of PIBCs and there was no evidence of unexpected harm. Although these results parallel those gathered in other clinical fields, further testing on SAH patients is required.[
A bimodal regimen of pharmaconutrition offers significant clinical advantages to provide n-3 PUFAs after SAH. Parenteral treatment can be efficiently delivered regardless of the patient's clinical condition and oral treatment can be easily extended to address novel therapeutic targets.[
The n-3 PUFAs dosage was supported by clinical evidence. The therapeutic dose range of FOLE (0.1 – 0.2 g FO/kg BW/day) is significantly lower than that of regular lipid emulsions.[
There was a lower occurrence of DCI-related outcomes and SDH in the IG than in the CG. Nevertheless, it would be erroneous to draw any conclusion about this from a small pilot trial that, consequently, was underpowered to detect any meaningful difference. The rate of consent was unexpectedly high in both centres. If the main drivers of acceptance were the less invasive nature of the intervention and the good reputation of n-3 PUFAs, the current trial should be widely replicable.[
Our pilot trial had several limitations. We did not define formal progression criteria to decide whether to proceed with a future definitive randomized controlled trial (RCT).[
To date, four clinical studies have investigated the role of n-3 PUFAs in SAH. Three of these studies reported an improved clinical outcome when oral n-3 PUFAs were compared with usual care.[
Although the balance between the benefit and harm of the intervention appears highly favourable, there are scarce data regarding the isolated use of FOLE as a specific treatment.[
Acknowledgments
We thank our patients and their families as well as the physicians, nurses, hospital pharmacists, nurse assistants, dieticians and administrative assistants who contributed to the execution of this trial. We thank Eugenio Flores for English language help.
Financial support and sponsorship
Funding was provided by the Public Health Care Service of the VI Region (Servicio de Salud O’Higgins). Exempt Resolution 1222/2013 and 1223/2013.
Conflicts of interest
There are no conflicts of interest.
References
1. Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010. 41: e519-36
2. Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006. 83: 1467S-76S
3. Badjatia N, Seres D, Carpenter A, Schmidt JM, Lee K, Mayer SA. Free Fatty acids and delayed cerebral ischemia after subarachnoid hemorrhage. Stroke. 2012. 43: 691-6
4. Bang HO, Dyerberg J, Nielsen AB. Plasma Lipid and Lipoprotein Pattern in Greenlandic West-Coast Eskimos. Nutr Rev. 2009. 44: 143-6
5. Bazan NG, Musto AE, Knott EJ. Endogenous signaling by omega-3 docosahexaenoic acid-derived mediators sustains homeostatic synaptic and circuitry integrity. Mol Neurobiol. 2011. 44: 216-22
6. Belayev L, Khoutorova L, Atkins KD, Eady TN, Hong S, Lu Y. Docosahexaenoic Acid therapy of experimental ischemic stroke. Transl Stroke Res. 2011. 2: 33-41
7. Belayev L, Mukherjee PK, Balaszczuk V, Calandria JM, Obenaus A, Khoutorova L. Neuroprotectin D1 upregulates Iduna expression and provides protection in cellular uncompensated oxidative stress and in experimental ischemic stroke. Cell Death Differ. 2017. 24: 1091-9
8. Bendel P, Koivisto T, Aikia M, Niskanen E, Kononen M, Hanninen T. Atrophic enlargement of CSF volume after subarachnoid hemorrhage: Correlation with neuropsychological outcome. AJNR Am J Neuroradiol. 2010. 31: 370-6
9. Bhatt A. Protocol deviation and violation. Perspect Clin Res. 2012. 3: 117-
10. Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology?. Br J Clin Pharmacol. 2013. 75: 645-62
11. Carter RE, Woolson RF. Statistical design considerations for pilot studies transitioning therapies from the bench to the bedside. J Transl Med. 2004. 2: 37-
12. Chen CT, Kitson AP, Hopperton KE, Domenichiello AF, Trepanier MO, Lin LE. Plasma non-esterified docosahexaenoic acid is the major pool supplying the brain. Sci Rep. 2015. 5: 15791-
13. De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med. 2011. 364: 2439-50
14. Deckelbaum RJ, Worgall TS, Seo T. n-3 fatty acids and gene expression. Am J Clin Nutr. 2006. 83: 1520S-5S
15. Doig GS, Simpson F. Randomization and allocation concealment: A practical guide for researchers. J Crit Care. 2005. 20: 187-91
16. Dyerberg J, Madsen P, Moller JM, Aardestrup I, Schmidt EB. Bioavailability of marine n-3 fatty acid formulations. Prostaglandins Leukot Essent Fatty Acids. 2010. 83: 137-41
17. Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ. 2016. 355: i5239-
18. Emanuel EJ, Wendler D, Grady C. What makes clinical research ethical?. JAMA. 2000. 283: 2701-11
19. Etminan N, Vergouwen MD, Ilodigwe D, Macdonald RL. Effect of pharmaceutical treatment on vasospasm, delayed cerebral ischemia, and clinical outcome in patients with aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. J Cereb Blood Flow Metab. 2011. 31: 1443-51
20. Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M. Omega-3 fatty acids: Evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006. 67: 1954-67
21. Grosso G, Pajak A, Marventano S, Castellano S, Galvano F, Bucolo C. Role of omega-3 fatty acids in the treatment of depressive disorders: A comprehensive meta-analysis of randomized clinical trials. PLoS One. 2014. 9: e96905-
22. Guo S, Lo EH. Dysfunctional cell-cell signaling in the neurovascular unit as a paradigm for central nervous system disease. Stroke. 2009. 40: S4-7
23. Hall TC, Bilku DK, Neal CP, Cooke J, Fisk HL, Calder PC. The impact of an omega-3 fatty acid rich lipid emulsion on fatty acid profiles in critically ill septic patients. Prostaglandins Leukot Essent Fatty Acids. 2016. 112: 1-11
24. Harris WS. Expert opinion: Omega-3 fatty acids and bleeding-cause for concern?. Am J Cardiol. 2007. 99: 44C-46C
25. Harris WS, Pottala JV, Sands SA, Jones PG. Comparison of the effects of fish and fish-oil capsules on the n 3 fatty acid content of blood cells and plasma phospholipids. Am J Clin Nutr. 2007. 86: 1621-5
26. He C, Qu X, Cui L, Wang J, Kang JX. Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc Natl Acad Sci U S A. 2009. 106: 11370-5
27. Heller AR, Rossler S, Litz RJ, Stehr SN, Heller SC, Koch R. Omega-3 fatty acids improve the diagnosis-related clinical outcome. Crit Care Med. 2006. 34: 972-9
28. Hibbeln JR, Nieminen LR, Blasbalg TL, Riggs JA, Lands WE. Healthy intakes of n-3 and n-6 fatty acids: Estimations considering worldwide diversity. Am J Clin Nutr. 2006. 83: 1483S-93S
29. Hijdra A, Brouwers PJ, Vermeulen M, van Gijn J. Grading the amount of blood on computed tomograms after subarachnoid hemorrhage. Stroke. 1990. 21: 1156-61
30. Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014. 348: g1687-
31. Hou J, Zhang JH. Does prevention of vasospasm in subarachnoid hemorrhage improve clinical outcome? No. Stroke. 2013. 44: S34-6
32. Hsieh YP, Lin CL, Shiue AL, Yin H, Morrow JD, Hsu JC. Correlation of F4-neuroprostanes levels in cerebrospinal fluid with outcome of aneurysmal subarachnoid hemorrhage in humans. Free Radic Biol Med. 2009. 47: 814-24
33. Jeansen S, Witkamp RF, Garthoff JA, van Helvoort A, Calder PC. Fish oil LC-PUFAs do not affect blood coagulation parameters and bleeding manifestations: Analysis of 8 clinical studies with selected patient groups on omega-3-enriched medical nutrition. Clin Nutr. 2017. p.
34. Jones NE, Heyland DK. Pharmaconutrition: A new emerging paradigm. Curr Opin Gastroenterol. 2008. 24: 215-22
35. Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D. Rapid appearance of resolvin precursors in inflammatory exudates: Novel mechanisms in resolution. J Immunol. 2008. 181: 8677-87
36. Kerkhoff LA, Butler J, Kelkar AA, Shore S, Speight CD, Wall LK. Trends in Consent for Clinical Trials in Cardiovascular Disease. J Am Heart Assoc. 2016. p. 5-
37. Kim HY. Novel metabolism of docosahexaenoic acid in neural cells. J Biol Chem. 2007. 282: 18661-5
38. Marik PE, Zaloga GP. Immunonutrition in critically ill patients: A systematic review and analysis of the literature. Intensive Care Med. 2008. 34: 1980-90
39. Massicotte EM, Del Bigio MR. Human arachnoid villi response to subarachnoid hemorrhage: Possible relationship to chronic hydrocephalus. J Neurosurg. 1999. 91: 80-4
40. McDougle DR, Watson JE, Abdeen AA, Adili R, Caputo MP, Krapf JE. Anti-inflammatory omega-3 endocannabinoid epoxides. Proc Natl Acad Sci U S A. 2017. 114: E6034-43
41. Michael-Titus AT, Priestley JV. Omega-3 fatty acids and traumatic neurological injury: From neuroprotection to neuroplasticity?. Trends Neurosci. 2014. 37: 30-8
42. Moore CG, Carter RE, Nietert PJ, Stewart PW. Recommendations for planning pilot studies in clinical and translational research. Clin Transl Sci. 2011. 4: 332-7
43. Naidech AM, Bendok BR, Bassin SL, Bernstein RA, Batjer HH, Bleck TP. Classification of cerebral infarction after subarachnoid hemorrhage impacts outcome. Neurosurgery. 2009. 64: 1052-7
44. Nakagawa I, Yokoyama S, Omoto K, Takeshima Y, Matsuda R, Nishimura F. omega-3 Fatty Acids Ethyl Esters Suppress Cerebral Vasospasm and Improve Clinical Outcome Following Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2017. 99: 457-64
45. Ni Choileain N, Redmond HP. Cell response to surgery. Arch Surg. 2006. 141: 1132-40
46. Niemoller TD, Bazan NG. Docosahexaenoic acid neurolipidomics. Prostaglandins Other Lipid Mediat. 2010. 91: 85-9
47. O’Kelly CJ, Kulkarni AV, Austin PC, Urbach D, Wallace MC. Shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage: Incidence, predictors, and revision rates. Clinical article. J Neurosurg. 2009. 111: 1029-35
48. Park T, Chen H, Kevala K, Lee JW, Kim HY. N-Docosahexaenoylethanolamine ameliorates LPS-induced neuroinflammation via cAMP/PKA-dependent signaling. J Neuroinflammation. 2016. 13: 284-
49. Pilitsis JG, Coplin WM, O’Regan MH, Wellwood JM, Diaz FG, Fairfax MR. Free fatty acids in human cerebrospinal fluid following subarachnoid hemorrhage and their potential role in vasospasm: A preliminary observation. J Neurosurg. 2002. 97: 272-9
50. Pottala JV, Yaffe K, Robinson JG, Espeland MA, Wallace R, Harris WS. Higher RBC EPA+DHA corresponds with larger total brain and hippocampal volumes: WHIMS-MRI study. Neurology. 2014. 82: 435-42
51. Rashid MA, Katakura M, Kharebava G, Kevala K, Kim HY. N-Docosahexaenoylethanolamine is a potent neurogenic factor for neural stem cell differentiation. J Neurochem. 2013. 125: 869-84
52. Ritz MA, Fraser R, Tam W, Dent J. Impacts and patterns of disturbed gastrointestinal function in critically ill patients. Am J Gastroenterol. 2000. 95: 3044-52
53. Schallner N, Pandit R, LeBlanc R, 3rd , Thomas AJ, Ogilvy CS, Zuckerbraun BS. Microglia regulate blood clearance in subarachnoid hemorrhage by heme oxygenase-1. J Clin Invest. 2015. 125: 2609-25
54. Schmidt R. Dose-finding studies in clinical drug development. Eur J Clin Pharmacol. 1988. 34: 15-9
55. Seifman MA, Lewis PM, Rosenfeld JV, Hwang PY. Postoperative intracranial haemorrhage: A review. Neurosurg Rev. 2011. 34: 393-407
56. Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008. 8: 349-61
57. Sgubin D, Aztiria E, Perin A, Longatti P, Leanza G. Activation of endogenous neural stem cells in the adult human brain following subarachnoid hemorrhage. J Neurosci Res. 2007. 85: 1647-55
58. Shen J, Huang KY, Zhu Y, Pan JW, Jiang H, Weng YX. Effect of statin treatment on vasospasm-related morbidity and functional outcome in patients with aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. J Neurosurg. 2017. 127: 291-301
59. Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood). 2008. 233: 674-88
60. Singer P, Shapiro H, Theilla M, Anbar R, Singer J, Cohen J. Anti-inflammatory properties of omega-3 fatty acids in critical illness: Novel mechanisms and an integrative perspective. Intensive Care Med. 2008. 34: 1580-92
61. Stonehouse W, Conlon CA, Podd J, Hill SR, Minihane AM, Haskell C. DHA supplementation improved both memory and reaction time in healthy young adults: A randomized controlled trial. Am J Clin Nutr. 2013. 97: 1134-43
62. Tam AK, Kapadia A, Ilodigwe D, Li Z, Schweizer TA, Macdonald RL. Impact of global cerebral atrophy on clinical outcome after subarachnoid hemorrhage. J Neurosurg. 2013. 119: 198-206
63. Terpolilli NA, Brem C, Buhler D, Plesnila N. Are We Barking Up the Wrong Vessels? Cerebral Microcirculation After Subarachnoid Hemorrhage. Stroke. 2015. 46: 3014-9
64. Tsekos E, Reuter C, Stehle P, Boeden G. Perioperative administration of parenteral fish oil supplements in a routine clinical setting improves patient outcome after major abdominal surgery. Clin Nutr. 2004. 23: 325-30
65. Umhau JC, Zhou W, Carson RE, Rapoport SI, Polozova A, Demar J. Imaging incorporation of circulating docosahexaenoic acid into the human brain using positron emission tomography. J Lipid Res. 2009. 50: 1259-68
66. van Heuven AW, Dorhout Mees SM, Algra A, Rinkel GJ. Validation of a prognostic subarachnoid hemorrhage grading scale derived directly from the Glasgow Coma Scale. Stroke. 2008. 39: 1347-8
67. Veldeman M, Hollig A, Clusmann H, Stevanovic A, Rossaint R, Coburn M. Delayed cerebral ischaemia prevention and treatment after aneurysmal subarachnoid haemorrhage: A systematic review. Br J Anaesth. 2016. 117: 17-40
68. Vergouwen MD, Etminan N, Ilodigwe D, Macdonald RL. Lower incidence of cerebral infarction correlates with improved functional outcome after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2011. 31: 1545-53
69. Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke. 2010. 41: 2391-95
70. von Schacky C. Omega-3 fatty acids in cardiovascular disease--an uphill battle. Prostaglandins Leukot Essent Fatty Acids. 2015. 92: 41-7
71. Waitzberg DL, Torrinhas RS. Fish oil lipid emulsions and immune response: What clinicians need to know. Nutr Clin Pract. 2009. 24: 487-99
72. Wartenberg KE, Mayer SA. Medical complications after subarachnoid hemorrhage. Neurosurg Clin N Am. 2010. 21: 325-38
73. Weitzel LR, Mayles WJ, Sandoval PA, Wischmeyer PE. Effects of pharmaconutrients on cellular dysfunction and the microcirculation in critical illness. Curr Opin Anaesthesiol. 2009. 22: 177-83
74. Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: Guidelines for their use. J Neurotrauma. 1998. 15: 573-85
75. Yang P, Chan D, Felix E, Cartwright C, Menter DG, Madden T. Formation and antiproliferative effect of prostaglandin E (3) from eicosapentaenoic acid in human lung cancer cells. J Lipid Res. 2004. 45: 1030-9
76. Yoneda H, Shirao S, Kurokawa T, Fujisawa H, Kato S, Suzuki M. Does eicosapentaenoic acid (EPA) inhibit cerebral vasospasm in patients after aneurysmal subarachnoid hemorrhage?. Acta Neurol Scand. 2008. 118: 54-9
77. Yoneda H, Shirao S, Nakagawara J, Ogasawara K, Tominaga T, Suzuki M. A prospective, multicenter, randomized study of the efficacy of eicosapentaenoic acid for cerebral vasospasm: The EVAS study. World Neurosurg. 2014. 81: 309-15
78. Yurko-Mauro K, McCarthy D, Rom D, Nelson EB, Ryan AS, Blackwell A. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 2010. 6: 456-64
79. Zhang M, Wang S, Mao L, Leak RK, Shi Y, Zhang W. Omega-3 fatty acids protect the brain against ischemic injury by activating Nrf2 and upregulating heme oxygenase 1. J Neurosci. 2014. 34: 1903-15
80. Zuijdgeest-van Leeuwen SD, Dagnelie PC, Rietveld T, van den Berg JW, Wilson JH. Incorporation and washout of orally administered n-3 fatty acid ethyl esters in different plasma lipid fractions. Br J Nutr. 1999. 82: 481-8
81. Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B. Improving the reporting of pragmatic trials: An extension of the CONSORT statement. BMJ. 2008. 337: a2390-