- Department of Neurosurgery, Maastricht University Medical Center, Maastricht, The Netherlands
- Department of School of Mental Health and Neurosciences, School of Mental Health and Neurosciences, Maastricht University, Maastricht, The Netherlands
- Academic Center for Epileptology, Maastricht University Medical Center, Maastricht, The Netherlands
- Department of Pathology, Maastricht University Medical Center, Maastricht, The Netherlands
- Department of Epileptology, Academic Center for Epileptology, Kempenhaeghe, Heeze, The Netherlands
Correspondence Address:
Olaf E. M. G. Schijns
Department of Neurosurgery, Maastricht University Medical Center, Maastricht, The Netherlands
Department of School of Mental Health and Neurosciences, School of Mental Health and Neurosciences, Maastricht University, Maastricht, The Netherlands
DOI:10.4103/2152-7806.179583
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: G. Schijns OE M, Beckervordersandforth J, Wagner L, Hoogland G. Long-term drug-resistant temporal lobe epilepsy associated with a mixed ganglioglioma and dysembryoplastic neuroepithelial tumor in an elderly patient. Surg Neurol Int 01-Apr-2016;7:
How to cite this URL: G. Schijns OE M, Beckervordersandforth J, Wagner L, Hoogland G. Long-term drug-resistant temporal lobe epilepsy associated with a mixed ganglioglioma and dysembryoplastic neuroepithelial tumor in an elderly patient. Surg Neurol Int 01-Apr-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/long%e2%80%91term-drug%e2%80%91resistant-temporal-lobe-epilepsy-associated-with-a-mixed-ganglioglioma-and-dysembryoplastic-neuroepithelial-tumor-in-an-elderly-patient/
Abstract
Background:Mixed ganglioglioma and dysembryoplastic neuroepithelial tumor (DNET) is an extremely rare neuropathological diagnosis. The sparse number of patients described are children or young adults with long-term drug-resistant epilepsy.
Case Description:We report on a rare case of this tumor in a 61-year-old patient with an epilepsy duration of almost 60 years. This patient received an epilepsy surgery work-up with the intention to cure his drug-resistant epilepsy by performing a complete lesionectomy. The available literature on these mixed tumors is reviewed.
Conclusion:A contrast-enhancing mixed ganglioglioma and DNET can mimic a malignant tumor and appears not only in children and young adults, but also in the elderly patients with chronic epilepsy. A long-lasting epilepsy, in this case almost 60 years, can be completely cured by a complete lesionectomy.
Keywords: Dysembryoplastic neuroepithelial tumor, ganglioglioma, glioneuronal tumor, surgery, temporal lobe epilepsy
INTRODUCTION
Gangliogliomas are the prototype of composite glioneuronal tumors, first described in 1926.[
Dysembryoplastic neuroepithelial tumors (DNETs) were first described in 1988.[
This case report describes the very rare combination of a mixed DNET-ganglioglioma in an elderly patient with a seizure duration of almost 60 years.
Since the first description of this tumor in 1997, only two patients (out of a total of 14), including this case, older than 50 years of age, have been described [
CASE REPORT
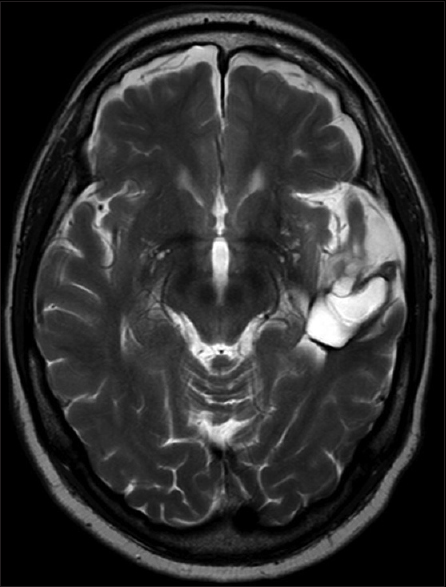
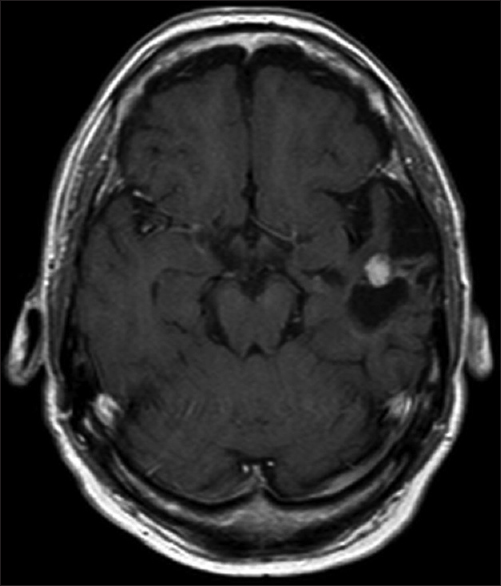
A 61-year-old man presented with chronic drug-resistant seizures since the age of four. After video-electroencephalogram (EEG) analysis, he was found to have complex partial seizures arising from the left temporal lobe. The seizures started with an unforced, nonversive head turning to the left, followed by a dystonic posturing of the right hand. Impairment of consciousness and apprehension together with dysphasia occurred. Postictally, the dysphasia continued and frequently there was nose wiping with the left hand. When he was considered a candidate for surgery, the patient had 1–2 seizures per month with a duration of 1 min. The seizures were treated with oxcarbazepine 1200 mg/day and valproic acid 1500 mg/day. The medical history was negative for epilepsy-associated risk factors such as febrile seizures, meningitis, and head trauma. The family history was negative with regard to epilepsy. Neurological examination demonstrated no abnormalities. A 3-Tesla magnetic resonance imaging (MRI) scan showed a cortical lesion in the medial and superior temporal gyrus of the anterior left temporal lobe, which appeared partially cystic and partially solid.
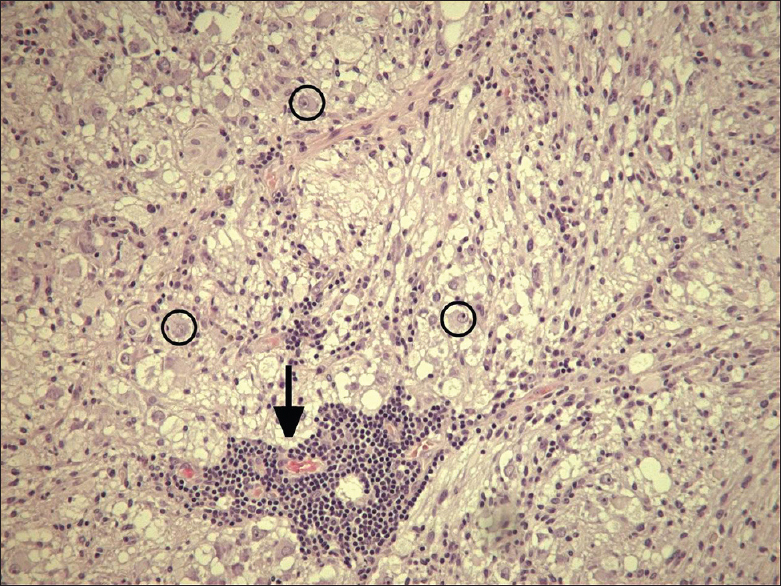
The solid part and the cyst wall enhanced after gadolinium administration. Signs of calcification or hemosiderin products were seen in the cyst wall [Figures
In the first two postoperative weeks, the patient had mixed speech disturbances most probably due to manipulation-induced edema. Three weeks after operation, these disturbances had disappeared completely. Until now (60 months after operation), the patient is seizure-free.
DISCUSSION
Glial tumors make up approximately half of the newly diagnosed primary brain tumors, with low-grade gliomas (LGGs) accounting for 15% of all brain tumors in adults.[
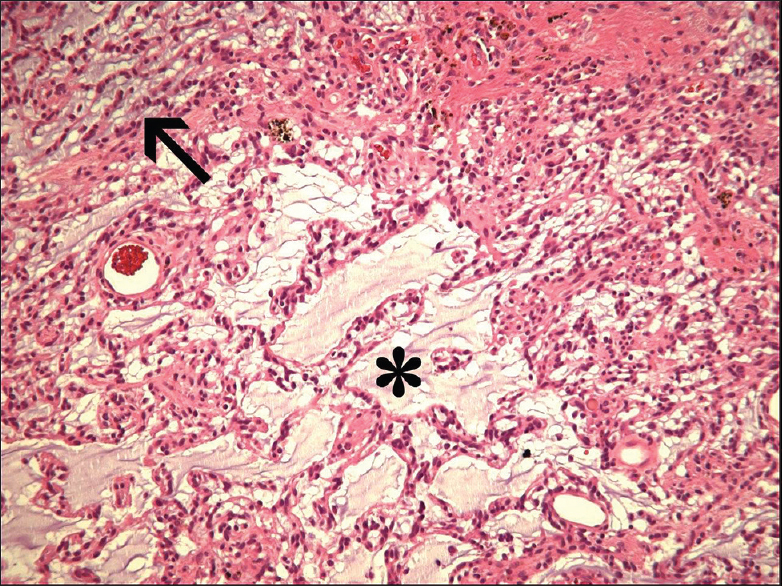
The first well-described example of a mixed ganglioglioma-DNET was the case report by Hirose et al. in 1998.[
This report describes the very rare case of a mixed ganglioglioma-DNET in an elderly patient; only one patient with an age above 50 was described earlier. A seizure duration of almost 60 years was not reported earlier. Most probably, the lesion was already present at the beginning of his seizures, 57 years ago. This shows once more the very indolent character of these tumors in the majority of patients and makes clear that a low-grade glioneuronal tumor always has to be included in the differential diagnosis of patients with long-term drug-resistant epilepsy, irrespective of their age. From a pragmatic, therapeutical point of view, it is important to realize that the ganglioglioma part of this mixed tumor, more frequently than the DNET component, can degenerate into an anaplastic or malignant form with a worse prognosis.
CONCLUSION
This and the fact that in some cases a third pathology, like FCD, is present leads to the conclusion that gross-total resection is the current best advice for these tumors with an optimal epileptological and oncological control as a dual aim.
This case demonstrates that an elderly patient with a long-lasting epilepsy and its associated epileptogenic network can become seizure-free (Engel IA) after complete lesionectomy of this rare mixed lesion of DNET with ganglioglioma. Resective surgery should be offered in comparable cases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Daumas-Duport C, Scheithauer BW, Chodkiewicz JP, Laws ER, Vedrenne C. Dysembryoplastic neuroepithelial tumor: A surgically curable tumor of young patients with intractable partial seizures. Report of thirty-nine cases. Neurosurgery. 1988. 23: 545-56
2. Davis GA, Kalnins RM, Fabinyi GC. Dysembryoplastic neuroepithelial tumour and mixed DNET-ganglioglioma: Seizure outcome following surgery. J Clin Neurosci. 1997. 4: 451-6
3. Guthrie BL, Laws ER. Supratentorial low-grade gliomas. Neurosurg Clin N Am. 1990. 1: 37-48
4. Hammond RR, Duggal N, Woulfe JM, Girvin JP. Malignant transformation of a dysembryoplastic neuroepithelial tumor. Case report. J Neurosurg. 2000. 92: 722-5
5. Hirose T, Scheithauer BW. Mixed dysembryoplastic neuroepithelial tumor and ganglioglioma. Acta Neuropathol. 1998. 95: 649-54
6. Lewis PD. Cell proliferation in the postnatal nervous system and its relationship to the origin of gliomas. Semin Neurol. 1981. 1: 181-7
7. Perkins OC. Ganglioglioma. Arch Pathol. 1926. 2: 11-7
8. Prayson RA, Estes ML, Morris HH. Coexistence of neoplasia and cortical dysplasia in patients presenting with seizures. Epilepsia. 1993. 34: 609-15
9. Prayson RA, Khajavi K, Comair YG. Cortical architectural abnormalities and MIB1 immunoreactivity in gangliogliomas: A study of 60 patients with intracranial tumors. J Neuropathol Exp Neurol. 1995. 54: 513-20
10. Prayson RA, Morris HH, Estes ML, Comair YG. Dysembryoplastic neuroepithelial tumor: A clinicopathologic and immunohistochemical study of 11 tumors including MIB1 immunoreactivity. Clin Neuropathol. 1996. 15: 47-53
11. Prayson RA, Napekoski KM. Composite ganglioglioma/dysembryoplastic neuroepithelial tumor: A clinicopathologic study of 8 cases. Hum Pathol. 2012. 43: 1113-8
12. Prayson RA. Composite ganglioglioma and dysembryoplastic neuroepithelial tumor. Arch Pathol Lab Med. 1999. 123: 247-50
13. Ray WZ, Blackburn SL, Casavilca-Zambrano S, Barrionuevo C, Orrego JE, Heinicke H. Clinicopathologic features of recurrent dysembryoplastic neuroepithelial tumor and rare malignant transformation: A report of 5 cases and review of the literature. J Neurooncol. 2009. 94: 283-92
14. Raymond AA, Halpin SF, Alsanjari N, Cook MJ, Kitchen ND, Fish DR. Dysembryoplastic neuroepithelial tumor. Features in 16 patients. Brain. 1994. 117: 461-75
15. Rushing EJ, Thompson LD, Mena H. Malignant transformation of a dysembryoplastic neuroepithelial tumor after radiation and chemotherapy. Ann Diagn Pathol. 2003. 7: 240-4
16. Shimbo Y, Takahashi H, Hayano M, Kumagai T, Kameyama S. Temporal lobe lesion demonstrating features of dysembryoplastic neuroepithelial tumor and ganglioglioma: A transitional form?. Clin Neuropathol. 1997. 16: 65-8
17. Taratuto AL, Pomata H, Sevlever G, Gallo G, Monges J. Dysembryoplastic neuroepithelial tumor: Morphological, immunocytochemical, and deoxyribonucleic acid analyses in a pediatric series. Neurosurgery. 1995. 36: 474-81