- Department of Neurological Surgery, Loyola University Medical Center, Maywood, Illinois
- Department of Otolaryngology, Loyola University Medical Center, Maywood, Illinois
- Department of Clinical Research Office Stritch School of Medicine, Loyola University Medical Center, Maywood, Illinois
- Department of Surgery, Edward Hines, Jr. VA Medical Center, Hines, Illinois, USA
Correspondence Address:
Anand V. Germanwala
Department of Neurological Surgery, Loyola University Medical Center, Maywood, Illinois
Department of Otolaryngology, Loyola University Medical Center, Maywood, Illinois
Department of Surgery, Edward Hines, Jr. VA Medical Center, Hines, Illinois, USA
DOI:10.25259/SNI-68-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nathan C. Pecoraro, Daniel M. Heiferman, Brendan Martin, Daphne Li, Stephen J. Johans, Chirag R. Patel, Anand V. Germanwala. Lower-dose perioperative steroid protocol during endoscopic endonasal pituitary adenoma resection. 24-Apr-2019;10:52
How to cite this URL: Nathan C. Pecoraro, Daniel M. Heiferman, Brendan Martin, Daphne Li, Stephen J. Johans, Chirag R. Patel, Anand V. Germanwala. Lower-dose perioperative steroid protocol during endoscopic endonasal pituitary adenoma resection. 24-Apr-2019;10:52. Available from: http://surgicalneurologyint.com/surgicalint-articles/9302/
Abstract
Background:Perioperative steroid management for pituitary adenoma resections is multifaceted due to possible hypothalamic–pituitary–adrenal (HPA) axis disruption. Although many different strategies have been proposed, there is no standard protocol for prophylaxis of potential hypocortisolemia.
Methods:We performed a retrospective analysis of consecutive endoscopic endonasal pituitary adenoma resections. Before March 2016, patients received ≥100 mg of hydrocortisone intraoperatively followed by 2 mg of dexamethasone immediately postoperatively in most of the patients. Subsequently, patients received only 50 mg of hydrocortisone intraoperatively. A morning cortisol level was checked on postoperative day (POD) 2, and if it was
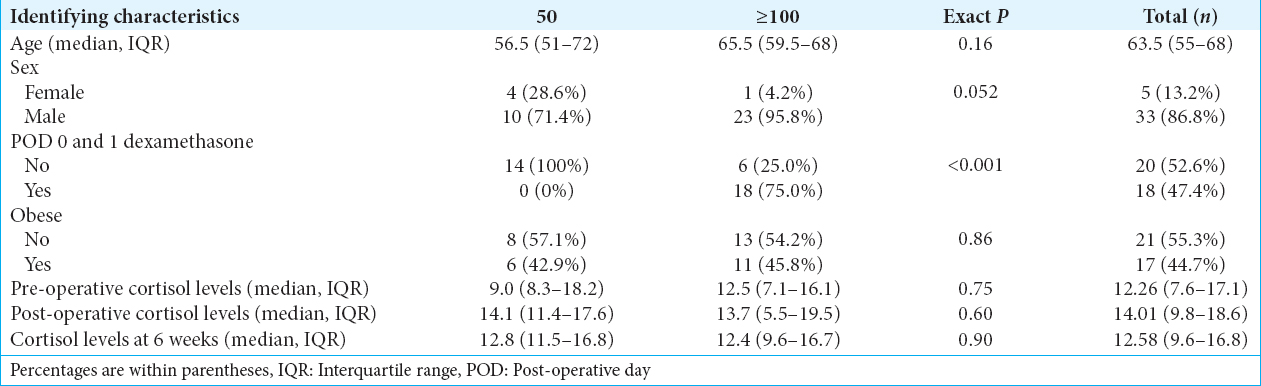
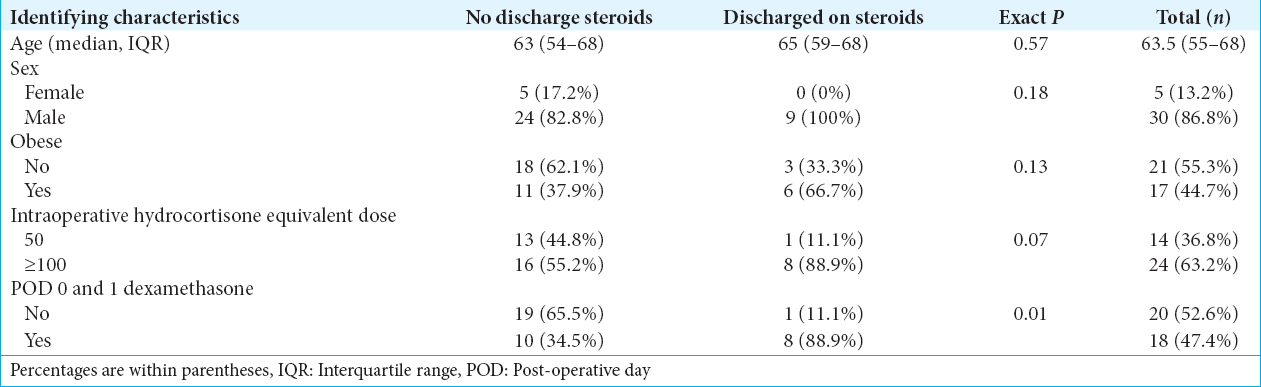
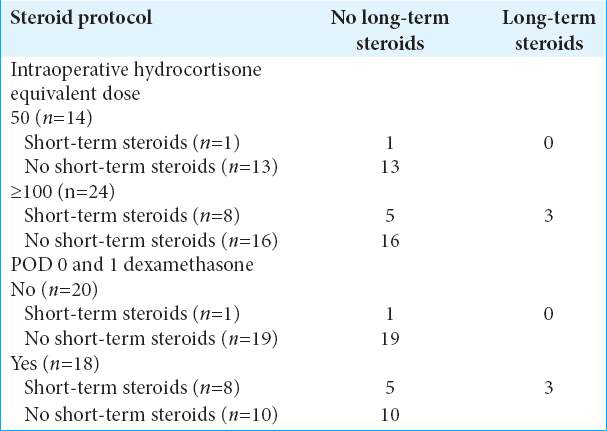
Results:Of those who received ≥100 mg of hydrocortisone, 8 of 24 (33.3%) were discharged on hydrocortisone compared to 1 of 14 (7.1%) who received 50 mg. 18 of 24 (75%) of ≥100 mg group received dexamethasone on POD 1, and of those, 8 (44.4%) were discharged on hydrocortisone. Of those who received ≥100 mg and were on outpatient steroid therapy initially, 3 of 8 (37.5%) required continuation after 6 weeks compared to none who received 50 mg. There was an association between patient’s intraoperative/immediate postoperative steroid use and steroid continuation at discharge.
Conclusion:Through our experience, we hypothesize that ≥100 mg of hydrocortisone intraoperatively followed by postoperative dexamethasone may be overly suppressive in patients with otherwise normally functioning HPA. A 50 mg intraoperative dose alone may be considered to lower rates of unnecessary steroid regimens postoperatively.
Keywords: Adrenal insufficiency, endoscopic endonasal, intraoperative hydrocortisone, pituitary adenoma, steroid replacement therapy, transsphenoidal resection
INTRODUCTION
In the 1950s, after Fraser and Lewis each published reports on mortalities related to intraoperative hemodynamic collapse of patients with chronic steroid use, “stress dose” steroid administration became pervasively implemented during the peri-operative period.[
Pituitary adenoma surgery offers unique challenges to find an optimized cortisol management strategy. As the “master gland,” the pituitary serves a crucial role in relaying afferent corticotropin-releasing hormone signals from the hypothalamus, through the hypothalamohypophyseal portal system, to the adrenal glands through adrenocorticotropic hormone (ACTH), first conceptualized by Cushing.[
Due to the dogmatic attention to perioperative stress dosing of steroids in patients at risk for hypoadrenalism, there has been significant work on the diagnosis of subclinical hypocortisolism in pituitary adenoma patients. However, there is a lack of consensus regarding which tests are regarded as an appropriate workup of subclinical hypocortisolism, with significant clinical limitations to each.[
While the risks associated with a short course of perioperative hydrocortisone are thought to be minimal, a question arose regarding the potential of transient adrenal suppression in an otherwise normally functioning HPA axis, resulting in an artificially low cortisol on postoperative evaluation. This concern came to light after a significant portion of patients were identified as needing outpatient steroid treatment, but at their 6-week follow-up retesting, no longer required chronic hydrocortisone therapy. We present our findings before and after changing our protocol from 100 mg of intraoperative hydrocortisone dose followed by two doses of postoperative dexamethasone to our new protocol of a single dose of 50 mg of intraoperative hydrocortisone administered during endoscopic endonasal pituitary adenoma resections, with no further steroid replacement therapy being administered postoperatively in patients with clinical and laboratory findings not suggestive of hypocortisolemia.
MATERIALS AND METHODS
Patients/procedure
We retrospectively analyzed consecutive patients over 18 years of age who underwent endoscopic endonasal transsphenoidal surgery for resection of a nonfunctioning sellar lesion over a 49-month period from January 2014 to February 2018. Patients with preoperative steroid use, pituitary apoplexy, preoperative panhypopituitarism, recurrent pituitary adenoma, or a final pathologic diagnosis other than pituitary adenoma were excluded from the study. All surgeries were performed in a technically uniform fashion by a single, dual-surgeon team (senior authors: A.V.G., neurosurgery, and C.R.P., otolaryngology) at either Loyola University Medical Center or Edward Hines, Jr. VA Medical Center. A single dose of intravenous hydrocortisone was administered before induction of anesthesia. Prior to March 2016, a dose of 100 mg of hydrocortisone was administered before induction of anesthesia. Following March 2016, a dose of 50 mg of hydrocortisone was administered. Most patients who received 100 mg of hydrocortisone were also postoperatively given 2-mg dexamethasone twice daily. This was held during the evening of postoperative day (POD) 1, resulting in this subset of patients receiving a total of two doses of dexamethasone postoperatively. Patients who received 50 mg of hydrocortisone and additional dexamethasone by anesthesia for airway management were included in the ≥100 mg group for analysis. Patients were monitored closely for clinical evidence of hypocortisolemia and treated accordingly, as indicated. This study was conducted with approval by the VA and University Hospitals’ Institutional Review Board [
Cortisol assessments
A total of three morning serum cortisol (MSC) levels were routinely measured: preoperatively, on POD2 morning, and at 6-week follow-up. If the patients had a post-operative MSC >10 μg/dL and did not have clinical evidence of hypocortisolism when evaluated by an endocrinologist, they were not given post-operative glucocorticoid replacement. If the POD2 MSC was ≤10 µg/dL, the patient was sent home on steroid replacement therapy (20 and 10 mg of hydrocortisone in the morning and at night, respectively).
Statistical analysis
Patients’ baseline demographic and clinical measures were compared by intraoperative hydrocortisone dose, as well as steroid continuation at discharge. Fisher’s exact tests were used for all categorical comparisons, while nonparametric Wilcoxon Rank Sum test was employed to assess continuous variables. An alpha error rate of P ≤ 0.05 was considered to be statistically significant. All statistical analyses were run using SAS 9.4 (Cary, NC).
Survey
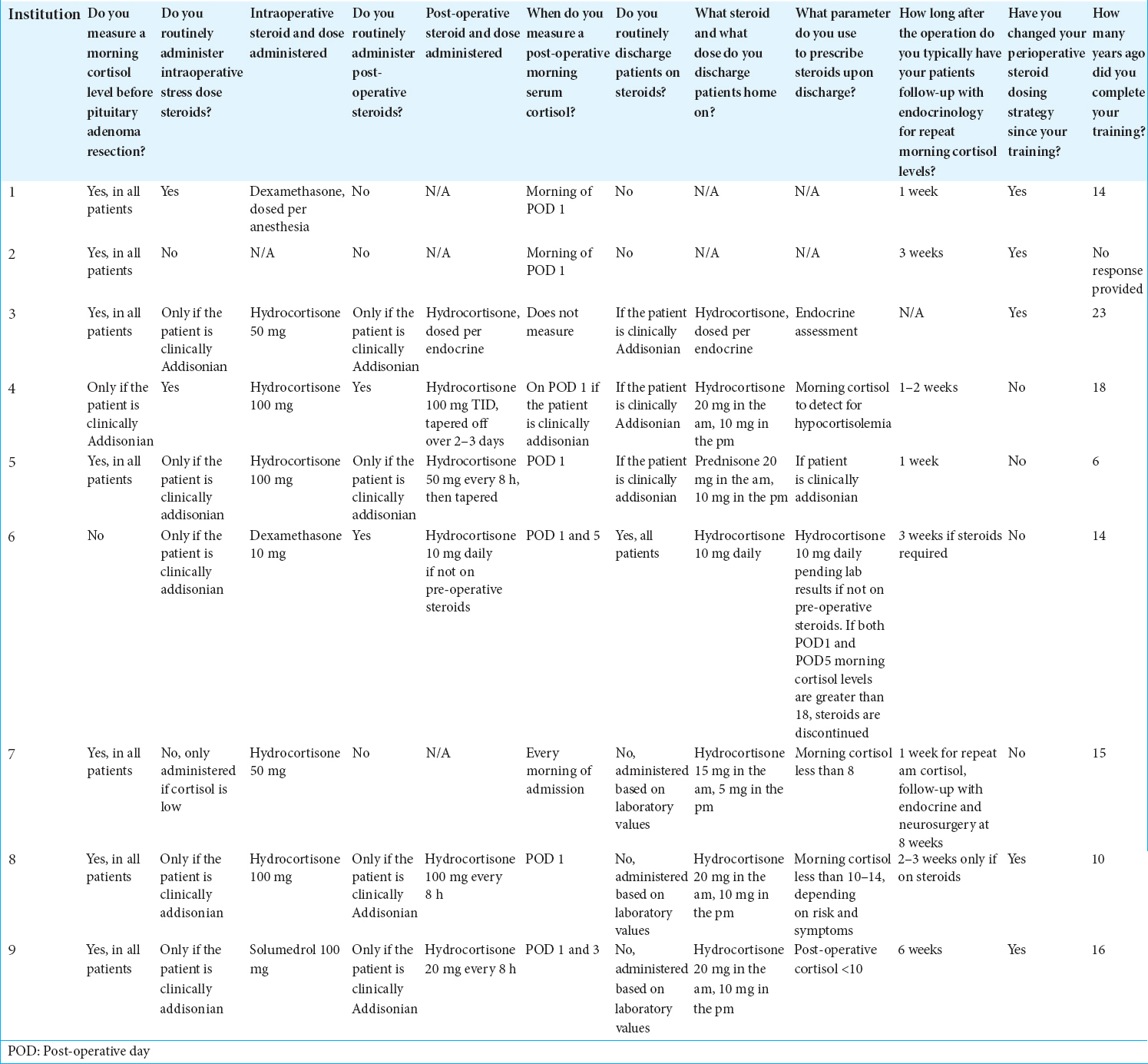
A sixteen-question survey was distributed to several US-based academic neurosurgeons who frequently perform endoscopic endonasal skull base surgery to shed light on the current perioperative hydrocortisone management protocols being employed nationally. Respondents were requested to base their answers on their practices regarding endoscopic endonasal resections of pituitary adenomas in patients with no prior history of pituitary adenoma surgery and not on pre-operative steroid replacement therapy. Questions investigated MSC testing, type and dosage of steroid medication used, indications for intra- and post-operative steroid therapy, and whether they had changed their protocol since their training.
RESULTS
Sixty-four patients underwent endoscopic endonasal transsphenoidal surgery for resection of a nonfunctioning sella lesion over the study period. 26 of these patients were excluded from the analysis due to final pathologic diagnosis other than pituitary adenoma (n = 4), pituitary apoplexy (n = 9), preoperative panhypopituitarism (n = 1), preoperative steroid use (n = 3), and recurrent pituitary adenoma (n = 6). Three patients had a combination of two or more exclusion criteria (n = 3). 38 patients were included in the analysis with 24 receiving ≥100 mg hydrocortisone or equivalent steroid and 14 receiving 50 mg. Of those that received ≥100 mg, 18 (75%) received 2 mg dexamethasone twice daily until being held at the night of POD1. Demographic information of the two cohorts is included in
All patients had POD2 MSC levels evaluated. A total of 10 patients had POD2 MSC <10 µg/dL (1.1–9.8 µg/dL), and all except one were placed on outpatient steroid replacement therapy per protocol. The patient with a POD2 MSC level of 9.8 µg/dL did not receive outpatient steroids. No other hormone deficiencies occurred.
Preliminary results suggest a marginal association between patients’ intraoperative and immediate postoperative steroid use with steroid use at discharge. Patients who received an intraoperative hydrocortisone dose of 50 mg were less likely to require steroids at discharge compared to patients who received ≥100 mg [P = 0.07,
Only three patients were continued on steroid replacement following an evaluation by an endocrinologist 6 weeks following surgery. Of those, all three had been operated on before March 2016 and had been given ≥100 mg intraoperatively followed by a short course of dexamethasone [
Survey results
A total of nine neurosurgeons responded to our survey. Reported practices were significantly heterogeneous [
DISCUSSION
The management of perioperative steroids for pituitary adenoma surgery remains a clinical challenge given a wide range of potential physiologic scenarios and proposed practices. Such has been demonstrated in our nationwide survey of academic neurosurgeons who routinely utilize endoscopic endonasal skull base techniques. Inder and Hunt and Wentworth et al. suggested that patients undergo a ACTH 1-24 (Synacthen) test.[
In addition to pre - and post-operative hypoadrenalism treatment, an intraoperative iatrogenic injury to the pituitary or infundibulum is a real, albeit uncommon, surgical complication. Thus, stress-dose steroid administration serves as a prophylactic measure to mediate such complications.[
The use of stress dose steroids and the particular dose are controversial. 100 mg of hydrocortisone was initially used as a standard stress dose intraoperatively, although this was likely leading to excessive transient adrenal suppression in our patients. It has been estimated that the physiologic cortisol levels in response to surgical stress range from 50 to 150 mg depending on the duration and systemic demands of the procedure.[
The retrospective nature of our study and nonrandomized cohorts resulted in some heterogeneity of our defined study groups, but we believe that pragmatic insights can still be drawn from our findings. Furthermore, with the limited sample size and our low surgical complication rate and a decreased need for outpatient steroid supplementations following resection of nonsecretory pituitary adenomas, a single intraoperative 50-mg hydrocortisone dose for preoperatively asymptomatic patients with normal MSC appears to be judicious. Further prospective investigation could provide more insight regarding the effects that a lower-dose intraoperative hydrocortisone strategy could have on the outcomes of patients undergoing pituitary adenoma resection.
CONCLUSION
Currently, a universally accepted protocol has yet to be established for hydrocortisone replacement therapy. A single intraoperative hydrocortisone replacement dose of 50 mg without postoperative steroid replacement therapy for nonaddisonian patients undergoing endoscopic endonasal resection of a pituitary adenoma is a safe and potentially beneficial protocol for perioperative adrenal insufficiency prophylaxis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Arafah BM. Reversible hypopituitarism in patients with large nonfunctioning pituitary adenomas. J Clin Endocrinol Metab. 1986. 62: 1173-9
2. Arraez MA. Assessment of postoperative hypocortisolism after pituitary surgery:When and how?. World Neurosurg. 2013. 80: 495-7
3. Auchus RJ, Shewbridge RK, Shepherd MD. Which patients benefit from provocative adrenal testing after transsphenoidal pituitary surgery?. Clin Endocrinol (Oxf). 1997. 46: 21-7
4. Ausiello JC, Bruce JN, Freda PU. Postoperative assessment of the patient after transsphenoidal pituitary surgery. Pituitary. 2008. 11: 391-401
5. Cappabianca P, Cavallo LM, Colao A, de Divitiis E. Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg. 2002. 97: 293-8
6. Cushing H.editorsThe Pituitary Body and its Disorders, Clinical States Produced by Disorders of the Hypophysis Cerebri. Philadelphia, PA, London: J.B. Lippincott Company; 1912. p.
7. De Tommasi C, Goguen J, Cusimano MD. Transphenoidal surgery without steroid replacement in patients with morning serum cortisol below 9 μg/dl (250 nmol/l). Acta Neurochir (Wien). 2012. 154: 1903-15
8. Ebersold MJ, Quast LM, Laws ER, Scheithauer B, Randall RV. Long-term results in transsphenoidal removal of nonfunctioning pituitary adenomas. J Neurosurg. 1986. 64: 713-9
9. Fleseriu M, Bodach ME, Tumialan LM, Bonert V, Oyesiku NM, Patil CG. Congress of neurological surgeons systematic review and evidence-based guideline for pretreatment endocrine evaluation of patients with nonfunctioning pituitary adenomas. Neurosurgery. 2016. 79: E527-9
10. Fraser CG, Preuss FS, Bigford WD. Adrenal atrophy and irreversible shock associated with cortisone therapy. J Am Med Assoc. 1952. 149: 1542-3
11. García-Luna PP, Leal-Cerro A, Rocha JL, Trujillo F, Garcia-Pesquera F, Astorga R. Evaluation of the pituitary-adrenal axis before, during and after pituitary adenomectomy. Is perioperative glucocorticoid therapy necessary?. Acta Endocrinol (Copenh). 1990. 122: 83-8
12. Hout WM, Arafah BM, Salazar R, Selman W. Evaluation of the hypothalamic-pituitary-adrenal axis immediately after pituitary adenomectomy:Is perioperative steroid therapy necessary?. J Clin Endocrinol Metab. 1988. 66: 1208-12
13. Inder WJ, Hunt PJ. Glucocorticoid replacement in pituitary surgery:Guidelines for perioperative assessment and management. J Clin Endocrinol Metab. 2002. 87: 2745-50
14. Jane JA, Laws ER. The surgical management of pituitary adenomas in a series of 3,093 patients. J Am Coll Surg. 2001. 193: 651-9
15. Jane JA, Thapar K, Kaptain GJ, Maartens N, Laws ER. Pituitary surgery:Transsphenoidal approach. Neurosurgery. 2002. 51: 435-42
16. Jayasena CN, Gadhvi KA, Gohel B, Martin NM, Mendoza N, Meeran K. Day 5 morning serum cortisol predicts hypothalamic-pituitary-adrenal function after transsphenoidal surgery for pituitary tumors. Clin Chem. 2009. 55: 972-7
17. Joseph SP, Ho JT, Doogue MP, Burt MG. Perioperative management of the hypothalamic-pituitary-adrenal axis in patients with pituitary adenomas:An Australasian survey. Intern Med J. 2012. 42: 1120-4
18. Kristof RA, Wichers M, Haun D, Redel L, Klingmüller D, Schramm J. Peri-operative glucocorticoid replacement therapy in transsphenoidal pituitary adenoma surgery:A prospective controlled study. Acta Neurochir (Wien). 2008. 150: 329-35
19. Lewis L, Robinson RF, Yee J, Hacker LA, Eisen G. Fatal adrenal cortical insufficiency precipitated by surgery during prolonged continuous cortisone treatment. Ann Intern Med. 1953. 39: 116-26
20. Marko NF, Gonugunta VA, Hamrahian AH, Usmani A, Mayberg MR, Weil RJ. Use of morning serum cortisol level after transsphenoidal resection of pituitary adenoma to predict the need for long-term glucocorticoid supplementation. J Neurosurg. 2009. 111: 540-4
21. Nemergut EC, Dumont AS, Barry UT, Laws ER. Perioperative management of patients undergoing transsphenoidal pituitary surgery. Anesth Analg. 2005. 101: 1170-81
22. Salem M, Tainsh RE, Bromberg J, Loriaux DL, Chernow B. Perioperative glucocorticoid coverage. A reassessment 42 years after emergence of a problem. Ann Surg. 1994. 219: 416-25
23. Tohti M, Li J, Zhou Y, Hu Y, Yu Z, Ma C. Is peri-operative steroid replacement therapy necessary for the pituitary adenomas treated with surgery?A systematic review and meta analysis. PLoS One. 2015. 10: e0119621-
24. Wentworth JM, Gao N, Sumithran KP, Maartens NF, Kaye AH, Colman PG. Prospective evaluation of a protocol for reduced glucocorticoid replacement in transsphenoidal pituitary adenomectomy:Prophylactic glucocorticoid replacement is seldom necessary. Clin Endocrinol (Oxf). 2008. 68: 29-35
25. Wilson CB, Dempsey LC. Transsphenoidal microsurgical removal of 250 pituitary adenomas. J Neurosurg. 1978. 48: 13-22
26. Zada G, Tirosh A, Huang AP, Laws ER, Woodmansee WW. The postoperative cortisol stress response following transsphenoidal pituitary surgery:A potential screening method for assessing preserved pituitary function. Pituitary. 2013. 16: 319-25
27. Zueger T, Kirchner P, Herren C, Fischli S, Zwahlen M, Christ E. Glucocorticoid replacement and mortality in patients with nonfunctioning pituitary adenoma. J Clin Endocrinol Metab. 2012. 97: E1938-42