- Department of Neurosurgery, Dubrava University Hospital, Zagreb, Croatia
- School of Medicine University of Zagreb, Zagreb, Croatia
- Department of Surgery, School of Medicine University of Zagreb, Zagreb, Croatia
- Department of Pathology and Cytology, Dubrava University Hospital, Zagreb, Croatia
Correspondence Address:
Marina Raguz, Department of Neurosurgery, University Hospital Dubrava, Zagreb, Croatia.
DOI:10.25259/SNI_120_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jurica Marakovic1,2, Darko Chudy1,3, Danko Muller2,4, Damir Tomac1, Petar Marcinkovic1, Darko Oreskovic1, Andjelo Kastelancic1, Marina Raguz1. Malignant transformation of pleomorphic xanthoastrocytoma in pregnant patient: Clinical case and ethical dilemma. 20-Sep-2021;12:469
How to cite this URL: Jurica Marakovic1,2, Darko Chudy1,3, Danko Muller2,4, Damir Tomac1, Petar Marcinkovic1, Darko Oreskovic1, Andjelo Kastelancic1, Marina Raguz1. Malignant transformation of pleomorphic xanthoastrocytoma in pregnant patient: Clinical case and ethical dilemma. 20-Sep-2021;12:469. Available from: https://surgicalneurologyint.com/surgicalint-articles/11123/
Abstract
Background: Pleomorphic xanthoastrocytoma (PXA) is a rare astrocytic tumor, accounting for
Case Description: A 28-year-old female patient was presented with a newly onset of headache, nausea, and right-sided hemiparesis at 21st week of pregnancy. Magnetic resonance imaging (MRI) revealed cystic mass in the left frontal region. Patient underwent biopsy to confirm pathohistological analysis; the tumor tissue corresponded to an anaplastic PXA. Two weeks after initial biopsy, open surgery along with gross total tumor removal was performed confirming pathohistological analysis. Six months later, after childbirth, and control MRI revealed a recurrent tumor mass: the patient underwent surgical resection and the tumor tissue corresponded to a glioblastoma. The patients were further treated with radiation and chemotherapy according to oncologist.
Conclusion: Distinguishing between PXA patients who have a good prognosis and those at risk for early progression is very important for the PXA clinical management. Despite cellular pleomorphism, mitotic index and the extent of resection are shown to be the main predictors for recurrence-free survival and overall survival rates. The standard therapy management is not yet established. Our patient treatment was associated with a significant ethical dilemma. Respecting patient’s wishes to deliver a baby, nor radio or chemo treatments were done. Further studies are necessary to provide factors responsible for malignant transformation of PXA. In addition, in ethically sensitive situation, such as tumor in pregnant patient, good communication, respecting patient’s wishes, and a multidisciplinary teamwork is the key for better outcome.
Keywords: Glioblastoma, Neurooncology, Pleomorphic xanthoastrocytoma, Pregnancy
INTRODUCTION
Pleomorphic xantho-astrocytoma (PXA) is a rare cerebral neoplasm first described in 1979 and added to the World Health Organization (WHO) classification of central nervous system neoplasms in 1993.[
Occurrence of malignant brain tumors, namely, gliomas, diagnosed during pregnancy is quite rare, and there are several cases describing treating management, along with associated ethical dilemmas.[
CASE DESCRIPTION
History
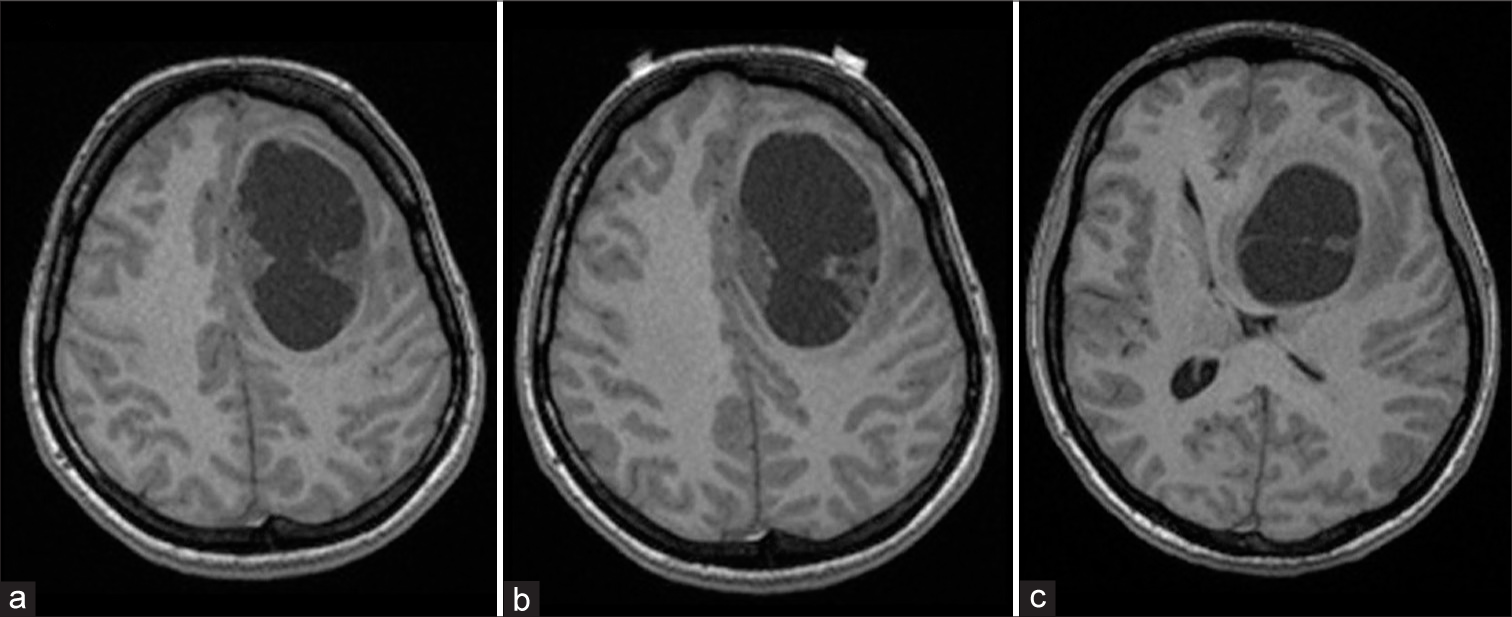
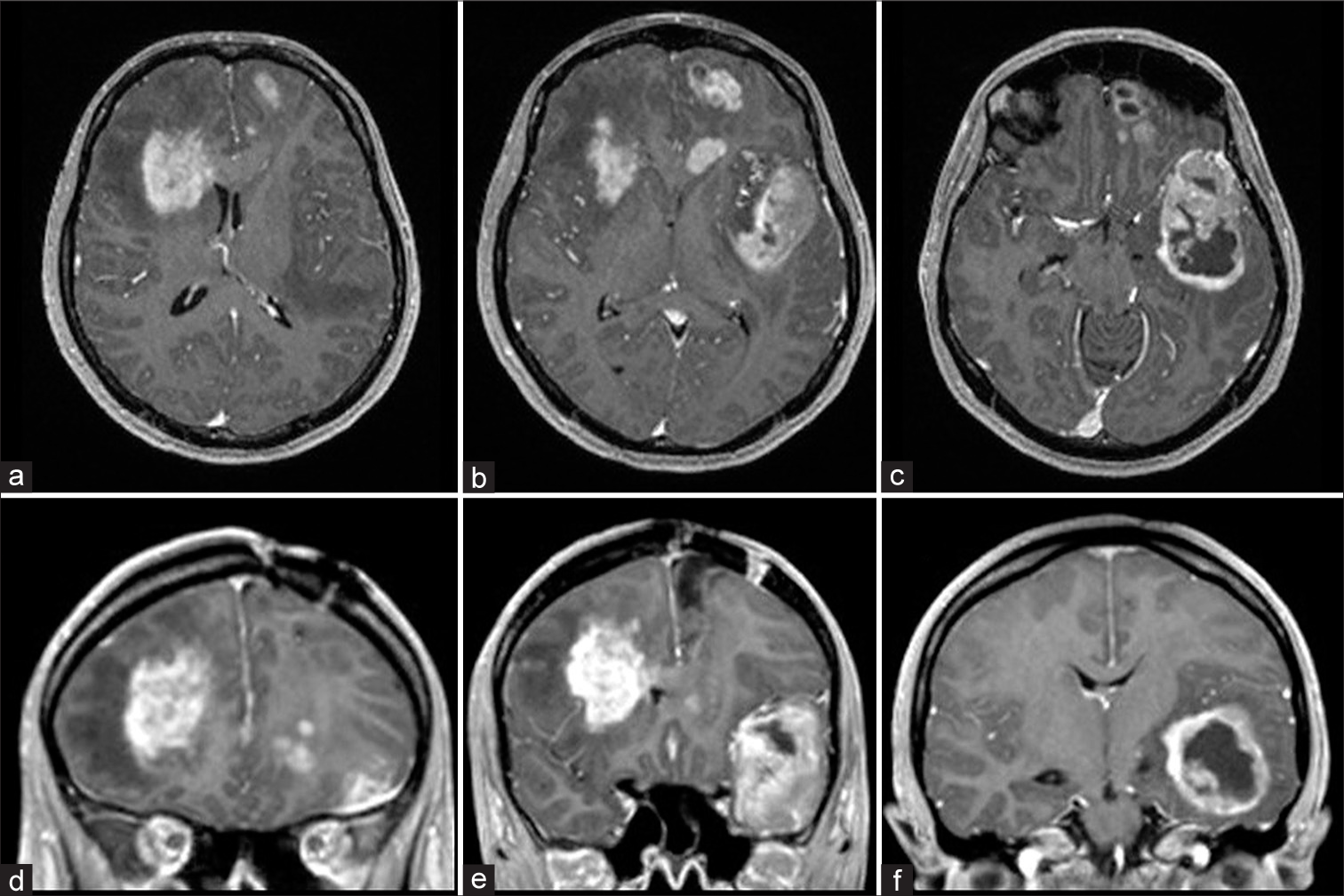
A 28-year-old female patient was presented with a newly onset of headache, nausea, and right-sided hemiparesis. No other symptoms nor neurological deficits were presented. The patient was 21 week pregnant. Patient had a history of grand mal epilepsy attacks and was on antiepileptic therapy since age of 12 till the age of 18; during that period, no neuroradiological scanning was performed. At the admission, carbamazepine therapy was prescribed by the neurologist. Initial magnetic resonance imaging (MRI) revealed cystic mass in the left frontal region; due to pregnancy intravenous contrast was not applied [
Operations and treatment
Patient underwent small left frontal osteoplastic craniotomy, evacuation of the cyst and the tumor biopsy under local anesthesia. Surgery went without any complications. Patient recovered uneventfully. Tissue samples acquired during the surgical procedure underwent pathohistological analysis. Since the pathohistological characteristics were challenging to interpret, both domestic and respectable foreign institutions were contacted and participated in analysis. Three types of tumor cells were noted; the most remarkable features are large polymorphic bizarre cells with large nuclei and atypical mitotic figures, followed by cells with empty vacuoles and small astrocytic like neoplastic cells in an epithelioid pattern. Parts of tumor consisted some reticulin network. A few pre-necrotic areas, collections of perivascular lymphocytes and areas with a slightly larger glial spindle cells were described. Microvascular proliferation was not observed. The tumor was strongly positive for SMI31 and 32 and SMI113, beta catenin, mainly on cellular membrane, as well as all neurofilament markers. Proliferation index was positive in 3–5% of the small cells, while in large, bizarre cells was around 30%. In addition, absence of the BRAF V600E mutation was showed. According to the WHO classification tumor tissue corresponded to an anaplastic PXA Grade III [
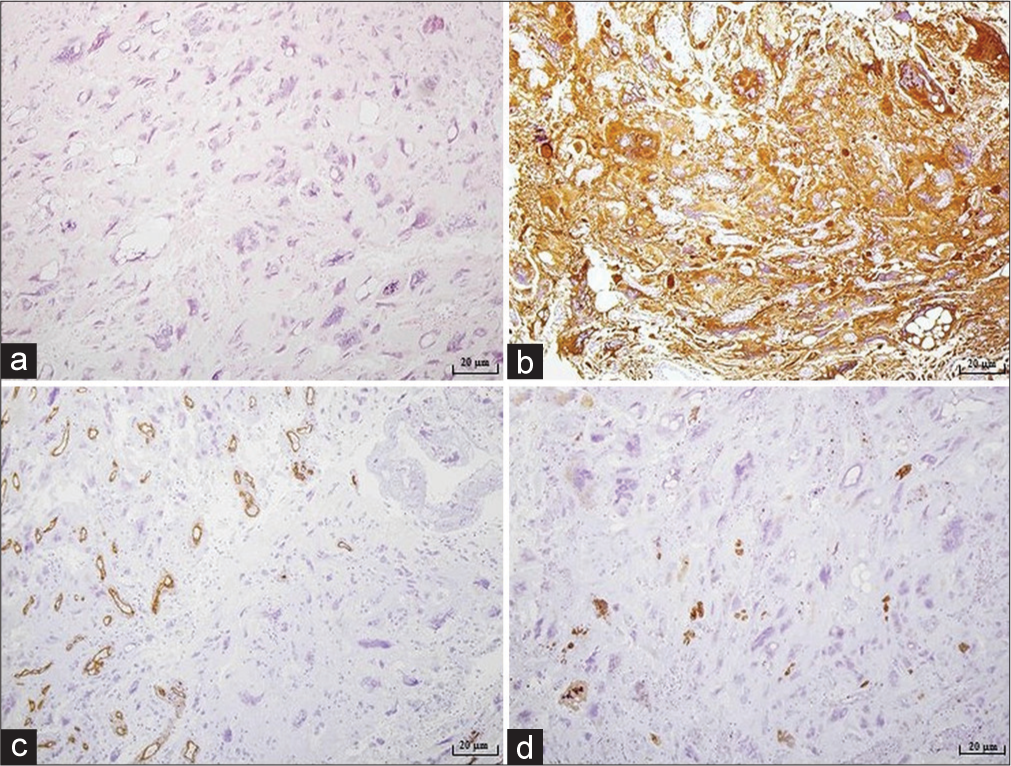
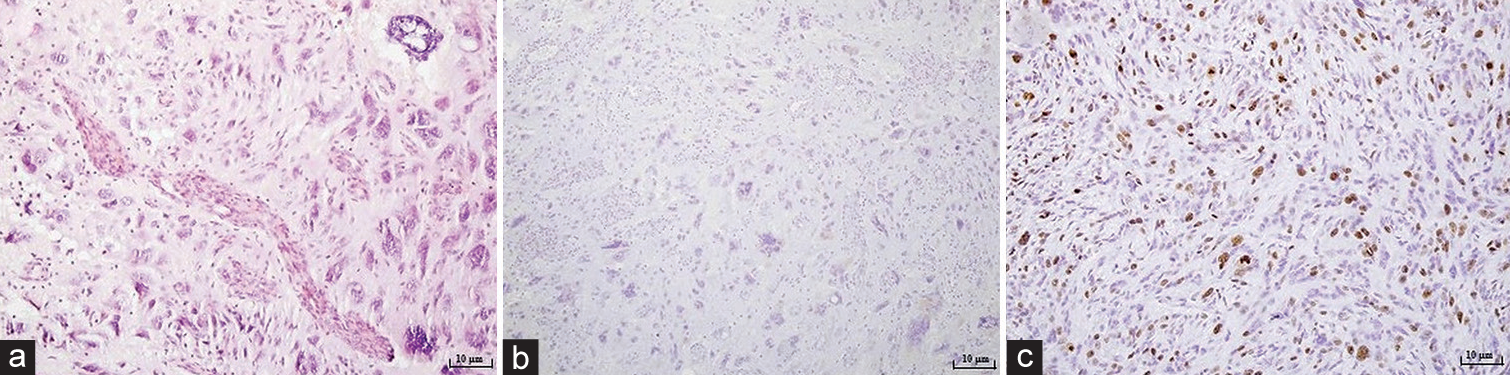
Figure 2:
Microphotography of a pathohistological section showing histological and immunohistochemical features of a tumorous tissue stained with (a) hematoxylin and eosin, original magnification of ×100, (b) GFAP, original magnification of ×100, (c) CD34, original magnification of ×100, (d) proliferation index Ki67, original magnification of ×100. Tumor consisted out of large polymorphic bizarre cells with large nuclei and atypical mitotic figures, cells with empty vacuoles and small astrocytic like neoplastic cells in an epithelioid pattern. A few pre-necrotic areas were described. Proliferation index was positive in 3–5% of the small cells, while in large, bizarre cells was around 30%. According to the WHO classification tumor tissue corresponded to a pleomorphic xanthoastrocytoma.
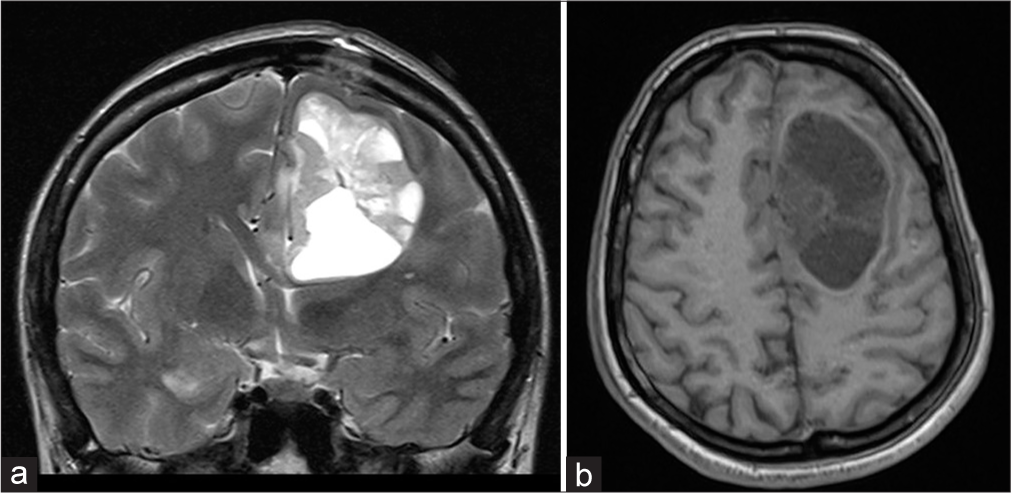
The patient, her husband and family were informed about diagnosis and treatment methods, including both chemo and radiotherapy, as well as the risk and anticipated complications that might happen during pregnancy. It was stated by the patient and her husband that they would like to carry on with the current pregnancy. New MRI was performed [
Six months later, after childbirth, control MRI revealed a supratentorial recurrent tumor mass in left frontal region with the cystic part in the superior frontal gyrus. After the administration of intravenous contrast, the mass showed heterogeneous enhancement [
Figure 5:
Microphotography of a pathohistological section showing histological and immunohistochemical features of a tumorous tissue stained with (a) hematoxylin and eosin, original magnification of ×100, (b) synuclein, original magnification of ×100, (c) proliferation index Ki67, original magnification of ×200. Tumor consisted out of atypical astroglial cells and high mitotic activity. Extensive microvascular proliferation of individual blood vessels was described, as well as focal points of tumor necrosis. Ki67 proliferation index higher was than 50%. According to the WHO classification, it corresponded glioblastoma multiforme, WHO Grade IV.
An oncologist was consulted. According to protocol for the treatment of primary glioma patient was referred to fractionated external beam radiation therapy of the whole brain (60 Gy) and temozolomide therapy. Furthermore, chemotherapy was administered. Two months after mentioned therapy, patient was further treated with monochemotherapy (temozolomide).
Postoperative course
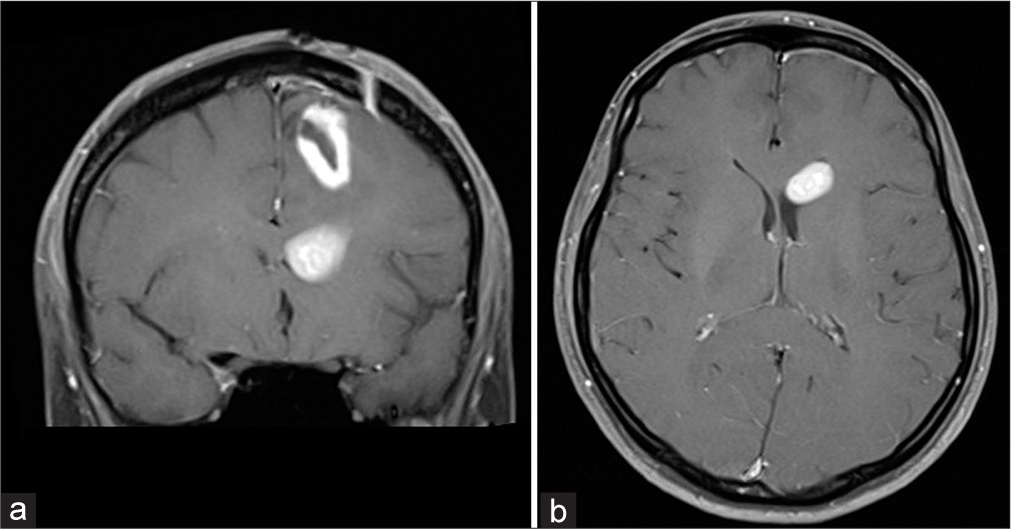
A year later, control MRI revealed postoperative cavity with pathological area of contrast accumulation in the posterior part, neuroradiologically described as probably residue of the tumor. No significant changes comparing to the previous MRI were found. Same findings were described yet on another control MRI after 6 months. Two year after initial diagnosis was established, patient, according to her husband’s claims, became disoriented and disturbed. She complained on a headache in frontal region. No significant neurological deficit was found. Computerized tomography scan showed hypodense zones in both, left and right, frontal region and in the left temporal region and narrowed lateral and third ventricles. MRI revealed expansive neoplastic masses in the right frontal region, left frontal region with rectal and orbital gyri and pericallosal area affected and in the left temporal region, along with necrotic zones [
DISCUSSION
Best to our knowledge, there has been no cases of malignant transformation of PXA to glioblastoma in pregnant patient in the literature. Several cases and small series describe individual approach in treating pregnant patients with glioma, but still, clear guidelines have not been established.[
Neurobiology of PXA is still controversial. Immunohistochemical studies support astrocytic and neuronal differentiation of PXA and indicate that PXA is probably a developmental neuroglial tumor with prominent glioproliferative changes associated with focal cortical dysplasia.[
According to the literature, PXA WHO Grade II behaves aggressively, recurring more frequently. During recurrence, its malignant potential is greater. Progression from the WHO Grade II to the WHO Grade IV has been previously described, frequently subsequently, but sometimes directly from Grade II to Grade IV.[
Due to very limited data and only few reported cases in the literature, we used the best available medical evidence to treat this patient. The multidisciplinary team which included the neurosurgeon, oncologist, and obstetrician discussed with the family all the risks and benefits of the pregnancy, the natural history of the disease and the possible outcome as well as the available treatment options. Following the course of pregnancy and knowing the patient and husband wishes, the risk of pregnancy versus benefits was fully explained. It was decided to fully support the mother during her pregnancy. Unfortunately, only 6 months after childbirth, MRI revealed recurrent tumor, and pathohistological diagnosis confirmed glioblastoma. Therefore, another gross total resection was performed, and oncologic treatment was administrated including both radio and chemo adjuvant therapy followed by monochemotherapy treatment with temozolomide, like presented in the literature.[
CONCLUSION
The rarity of PXA demands neuropathologic experience to find the correct diagnosis, since misinterpretation might cause harmful therapeutically decisions. A close follow-up is needed in order to detect any recurrence with malignant transformation. Furthermore, PXA probably originates in genetic changes which lead to its unpredictable biological behavior. Further molecular studies are necessary in order to elucidate the malignant transformation cascade in PXAs and eventually allow for genetic testing of these tumors after initial resection to provide insight on prognosis and need for adjuvant therapy. PXA diagnosis in pregnancy is a medical and ethical dilemma and generally carries a variable prognosis due to tumor pleomorphism. The multidisciplinary approach and frequent family counseling are important factors for managing patient and potentially successful treatment outcome.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent. Patients family has given informed consent for participation in this paper.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent. Patients family has given informed consent for participation in this paper.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Al-Rasheedy IM, Al-Hameed FM. Advanced case of glioblastoma multiforme and pregnancy: An ethical dilemma. Neurosciences (Riyadh). 2015. 20: 388-91
2. Binesh F, Akhavan A, Navabii H. Pleomorphic xanthoastrocytoma with malignant transformation and multiple recurrences in an Iranian girl. BMJ Case Rep. 2012. 2012: bcr1220115372
3. Chakrabarty A, Mitchell P, Bridges LR, Franks AJ. Malignant transformation in pleomorphic xanthoastrocytoma-a report of two cases. Br J Neurosurg. 1999. 13: 516-9
4. Flechl B, Hassler MR, Kopetzky G, Balcke P, Kurz C, Marosi C. Case report: Pregnancy in a patient with recurrent glioblastoma. F1000Res. 2013. 2: 246
5. Fouladi M, Jenkins J, Burger P, Langston J, Merchant T, Heideman R. Pleomorphic xanthoastrocytoma: Favorable outcome after complete surgical resection. Neuro Oncol. 2001. 3: 184-92
6. Furuta T, Miyoshi H, Komaki S, Arakawa F, Morioka M, Ohshima K. Clinicopathological and genetic association between epithelioid glioblastoma and pleomorphic xanthoastrocytoma. Neuropathology. 2018. 38: 218-27
7. Gelpi E, Popovic M, Preusser M, Budka H, Hainfellner J. Pleomorphic xanthoastrocytoma with anaplastic features presenting without GFAP immunoreactivity: Implications for differential diagnosis. Neuropathology. 2005. 25: 241-6
8. Ida CM, Rodriguez FJ, Burger PC, Caron AA, Jenkins SM, Spears GM. Pleomorphic xanthoastrocytoma: Natural history and long-term follow-up. Brain Pathol. 2015. 25: 575-86
9. Kanamori M, Suzuki H, Takei H, Sonoda Y, Uenohara H, Tominaga T. Malignant transformation of diffuse astrocytoma to glioblastoma associated with newly developed BRAF V600E mutation. Brain Tumor Pathol. 2016. 33: 50-6
10. Kaulich K, Blaschke B, Numann A, von Deimling A, Wiestler OD, Weber RG. Genetic alterations commonly found in diffusely infiltrating cerebral gliomas are rare or absent in pleomorphic xanthoastrocytomas. J Neuropathol Exp Neurol. 2002. 61: 1092-9
11. Kepes JJ, Rubinstein LJ, Eng LF. Pleomorphic xanthoastrocytoma: A distinctive meningocerebral glioma of young subjects with relatively favorable prognosis. A study of 12 cases. Cancer. 1979. 44: 1839-52
12. Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D, Cavenee WK. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
13. Lubansu A, Rorive S, David P, Sariban E, Seligmann R, Brotchi J. Cerebral anaplastic pleomorphic xanthoastrocytoma with meningeal dissemination at first presentation. Childs Nerv Syst. 2004. 20: 119-22
14. Macaulay RJ, Jay V, Hoffman HJ, Becker LE. Increased mitotic activity as a negative prognostic indicator in pleomorphic xanthoastrocytoma. Case report. J Neurosurg. 1993. 79: 761-8
15. Nakajima T, Kumabe T, Shamoto H, Watanabe M, Suzuki H, Tominaga T. Malignant transformation of pleomorphic xanthoastrocytoma. Acta Neurochir (Wien). 2006. 148: 67-1
16. She D, Liu J, Xing Z, Zhang Y, Cao D, Zhang Z. MR imaging features of anaplastic pleomorphic xanthoastrocytoma mimicking high-grade astrocytoma. AJNR Am J Neuroradiol. 2018. 39: 1446-52
17. van Westrhenen A, Senders JT, Martin E, DiRisio AC, Broekman ML. Clinical challenges of glioma and pregnancy: A systematic review. J Neurooncol. 2018. 139: 1-11
18. Vu TM, Liubinas SV, Gonzales M, Drummond KJ. Malignant potential of pleomorphic xanthoastrocytoma. J Clin Neurosci. 2012. 19: 12-20
19. Wu J, Ma YH, Wang TL. Glioma in the third trimester of pregnancy: Two cases and a review of the literature. Oncol Lett. 2013. 5: 943-6
20. Yamada SM, Murakami H, Tomita Y, Nakane M, Shibui S, Takahashi M. Glioblastoma multiforme versus pleomorphic xanthoastrocytoma with anaplastic features in the pathological diagnosis: A case report. Diagn Pathol. 2016. 11: 65