- Department of Neurosurgery, National Defense Medical College, Tokorozawa, Saitama, Japan
Correspondence Address:
Naoki Otani
Department of Neurosurgery, National Defense Medical College, Tokorozawa, Saitama, Japan
DOI:10.4103/sni.sni_124_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Naoki Otani, Terushige Toyooka, Satoru Takeuchi, Arata Tomiyama, Kojiro Wada, Kentaro Mori. Modified extradural temporopolar approach with mini-peeling of dura propria for paraclinoid and/or parasellar tumors: Operative technique and nuances. 22-Aug-2017;8:199
How to cite this URL: Naoki Otani, Terushige Toyooka, Satoru Takeuchi, Arata Tomiyama, Kojiro Wada, Kentaro Mori. Modified extradural temporopolar approach with mini-peeling of dura propria for paraclinoid and/or parasellar tumors: Operative technique and nuances. 22-Aug-2017;8:199. Available from: http://surgicalneurologyint.com/surgicalint-articles/modified-extradural-temporopolar-approach-with-mini%e2%80%91peeling-of-dura-propria-for-paraclinoid-andor-parasellar-tumors-operative-technique-and-nuances/
Abstract
Background:Modified extradural temporopolar approach (EDTPA) with mini-peeling of the dura propria can provide extensive exposure of the anterior clinoid process and early exposure, as well as complete mobilization and decompression of the optic nerve and internal carotid artery, which can prevent intraoperative neurovascular injury for paraclinoid and/or parasellar lesions. The present study investigated the usefulness of this modified technique and discusses the operative nuances.
Methods:We retrospectively reviewed medical charts of 27 consecutive patients with neoplastic paraclinoid and/or parasellar lesions who underwent this modified approach between September 2009 and August 2016.
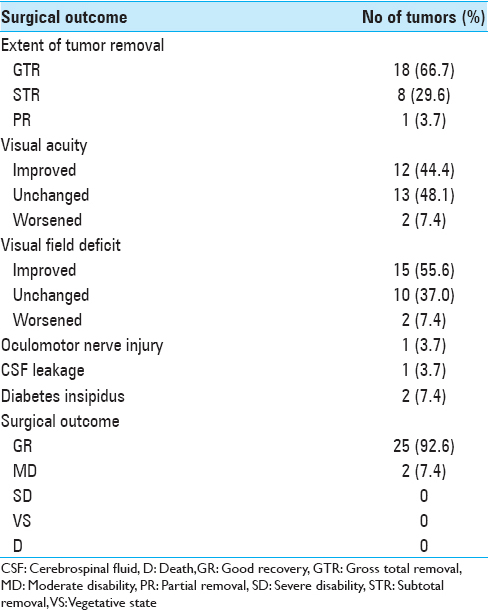
Results:Preoperative visual acuity worsened in 2 patients (7.4%), and worsening of visual field function occurred in 2 patients (7.4%). Postoperative outcome was good recovery in 25 patients (92.6%) and moderate disability in 2 (7.4%). No operation-related mortality occurred in the series.
Conclusions:The modified EDTPA is safe and recommended for surgical treatment of paraclinoid and/or parasellar tumors to reduce the risk of intraoperative optic neurovascular injury.
Keywords: Extradural temporopolar approach, microneurosurgery, paraclinoid tumor, skull base surgery
INTRODUCTION
Tumors in the paraclinoid and/or parasellar areas are still challenging to resect completely and safely because of their tendency to adhere to the optic apparatus, pituitary stalk, or internal carotid artery (ICA), as well as to invade the cavernous sinus (CS), orbit, and sella turcica.[
The extradural technique for complete removal of the ACP was first introduced by Dolenc in 1983,[
The present study demonstrated the usefulness of this modified EDTPA and its operative techniques, and evaluated the surgical outcomes with special emphasis on the clinical and visual outcomes after surgical resection of paraclinoid and/or parasellar tumors in a series of 27 consecutive patients.
PATIENTS AND METHODS
Patient characteristics
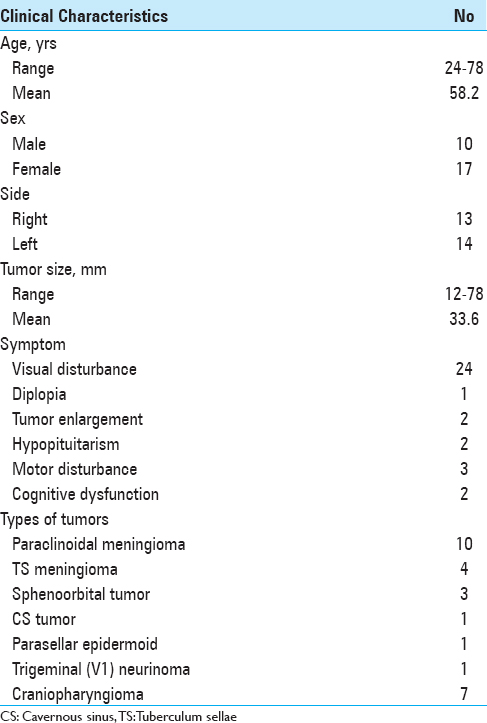
This retrospective analysis included 27 consecutive patients, 17 women and 10 men aged 24–78 years (mean, 58.2 years), with neoplastic lesions who underwent modified EDTPA with mini-peeling of the dura propria at the National Defense Medical College Hospital and Juntendo University Shizuoka Hospital between September 2009 and August 2015. Medical charts, radiological findings, surgical techniques, complications, and final results were retrospectively reviewed. The topography of all tumors was analyzed accurately using preoperative magnetic resonance (MR) imaging and intraoperative surgical assessment. Tumor size, obtained by measuring the greatest diameter in any single plane on the preoperative MR images, ranged from 12 to 78 mm (mean, 33.6 mm). The ACP size, shape, pneumatization, and relationship with the sphenoid or ethmoid sinus were assessed on bone computed tomography (CT) scans for safe clinoidectomy. The presence of contraindications for extradural anterior clinoidectomy, such as caroticoclinoid foramen and interclinoid osseous ligament, was also assessed preoperatively. The extent of tumor removal was based on intraoperative assessment and contrast-enhanced CT and/or MR imaging performed on the day after surgery. All patients underwent detailed pre and postoperative evaluation of visual function by a neuro-ophthalmologist, except for 2 patients with severe disorientation. Ophthalmological examinations consisted of testing visual acuity with optimum correction lenses for both eyes, visual field examinations, and funduscopy.
Surgical procedure
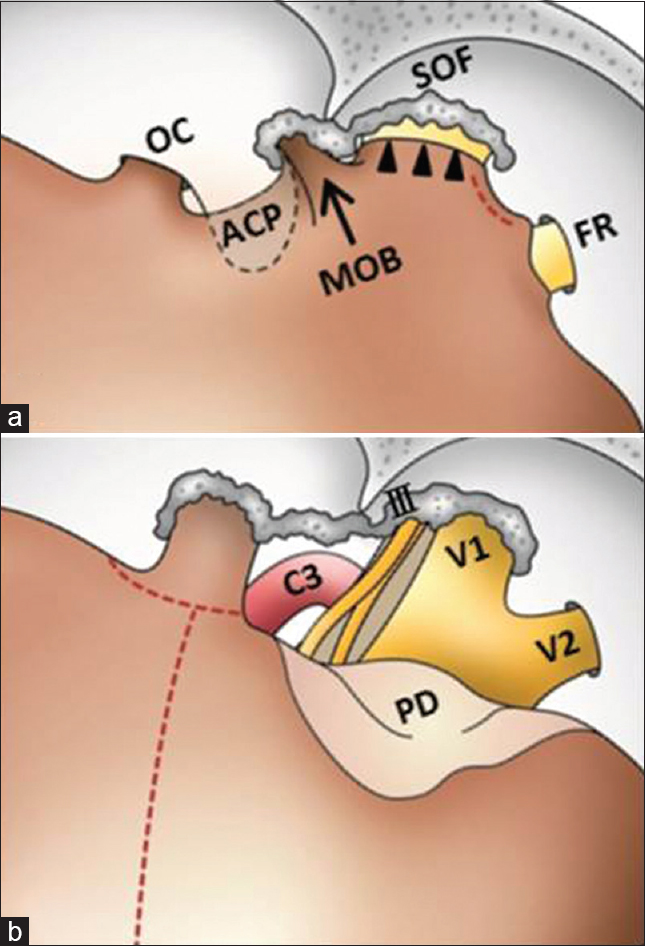
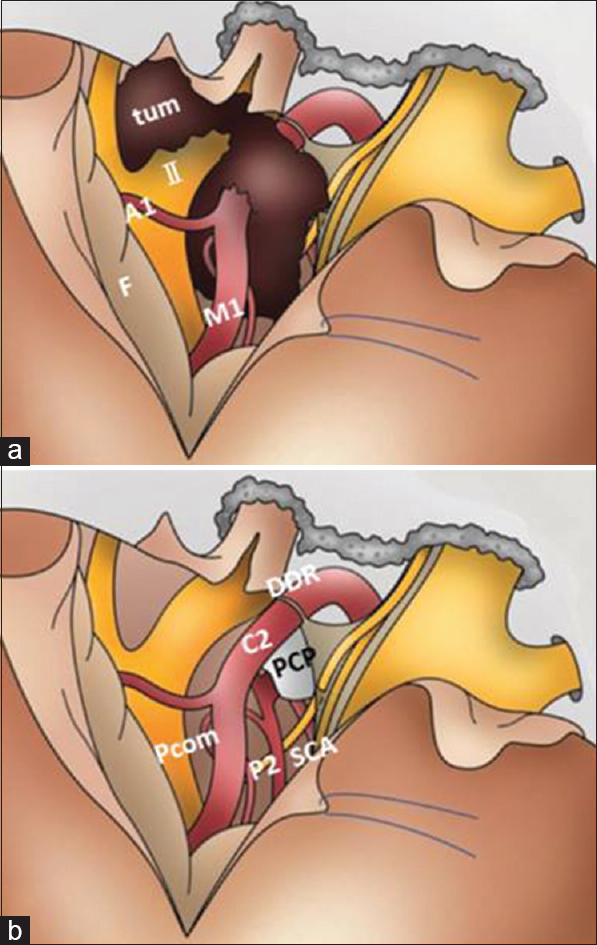
Schematic illustrations show the surgical procedure in Figures
The dura mater is opened along the sylvian fissure, and continued inferomedially to the level of the optic nerve [
Figure 2
Surgical procedures for removal of paraclinoid and/or parasellar tumors using the modified EDTPA technique. A1 = horizontal portion of the ACA; C2 = C2 portion of the ICA; F = frontal lobe; M1 = horizontal portion of the MCA; P2 = P2 portion of the PCA; Pcom = posterior communicating artery; PCP = posterior clinoid process; SCA = superior cerebellar artery; tum = tumor; DDR; distal dural ring
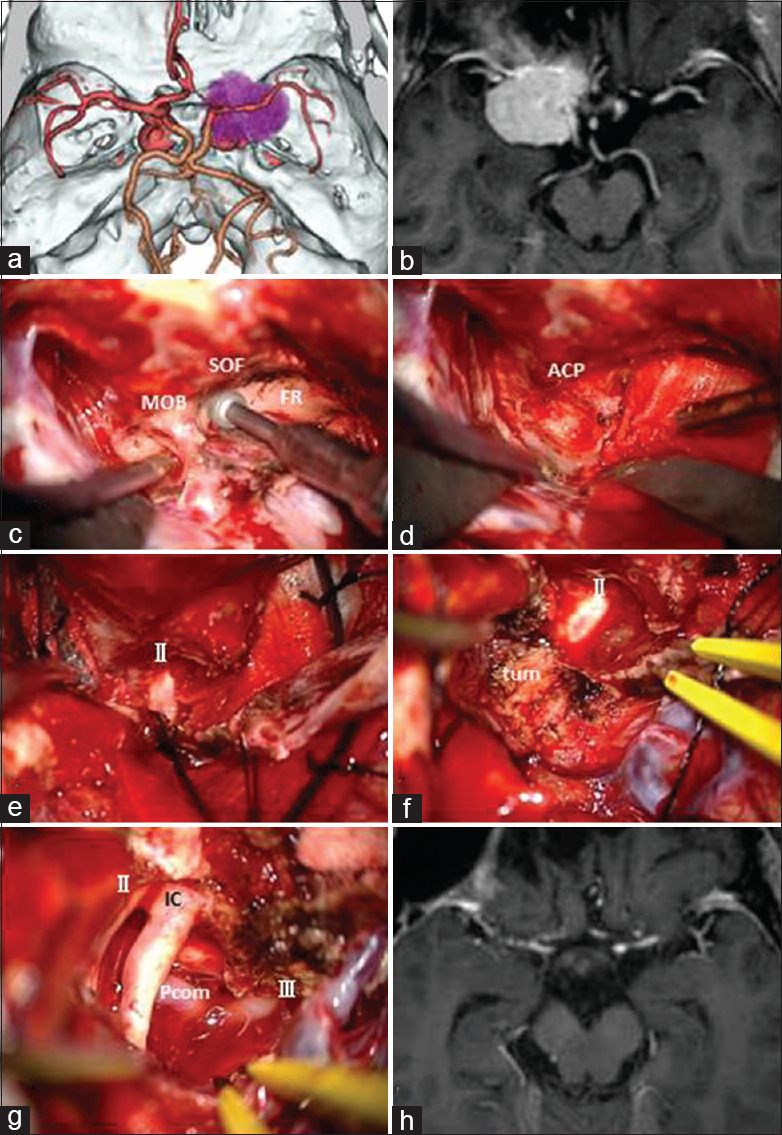
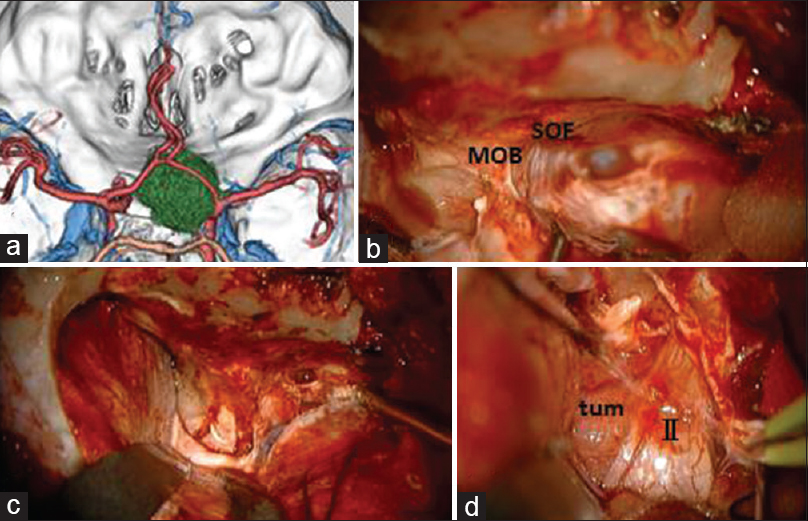
For removal of anterior skull base tumors such as paraclinoid meningioma, after opening of the sylvian fissure, dural detachment and devascularization can be completely and safely performed. After identification of the ipsilateral optic nerve and ICA, the tumor capsule is opened and the tumor is internally decompressed with ultrasonic surgical aspiration. After internal decompression and reduction of the tumor volume, the ipsilateral optic nerve and chiasm are decompressed by sharp dissection between the tumor capsule and the arachnoid membrane. After identification of the contralateral optic nerve, the site of the dural attachment is progressively and completely coagulated with bipolar coagulating forceps to interrupt the blood supply. The bilateral olfactory nerves can be preserved anatomically. The superior pole of the tumor can be reduced from beneath the arachnoid membrane covering the ipsilateral anterior cerebral artery. After additional piecemeal debulking of the tumor, the tumor located underneath the optic chiasm can be removed in the contralateral to the ipsilateral direction and then from beneath the ipsilateral optic nerve and ICA. Finally, the tumor can be dissected from the pituitary stalk and anatomical vital structures located in the interpeduncular cistern [
RESULTS
Patient characteristics
The clinical characteristics of the patients are summarized in
Surgical procedures performed
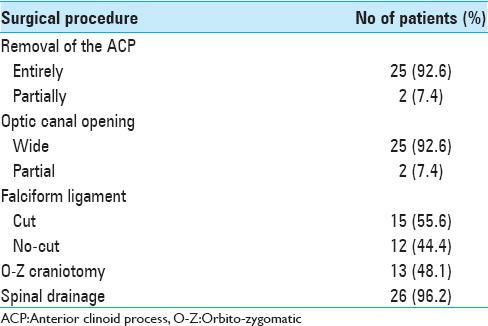
The surgical procedures are summarized in
Surgical and visual outcome
The surgical outcome is summarized in
Complications related to modified EDTPA
Complications are summarized in
Representative cases
Case 1: A 68-year-old female presented with right progressive visual disturbance. Three-dimensional CT angiography showed a tumor adjacent to the ACP [
Case 2: A 42-year-old male presented with right progressive visual disturbance. Three-dimensional CT angiography showed a tumor adjacent to the ACP [
DISCUSSION
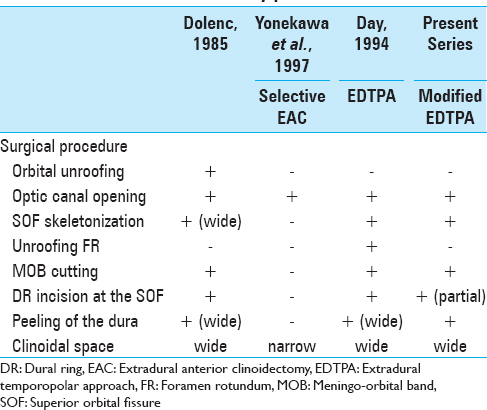
We have modified the original EDTPA to establish a less invasive transcavernous approach. Our modifications consist of skeletonization of the SOF, small dura propria incision between SOF and FR where no neurovascular structure is present, and limited dura propria peeling up to the V2 adequate to expose the entire ACP epidurally. In the present study, we applied our modified EDTPA to 27 cases of paraclinoid and/or parasellar tumors, resulting in relatively high removal rate and low incidence of cranial nerve disturbances such as visual disturbance, oculomotor nerve palsy, and trigeminal nerve dysfunction.
Technical considerations and advantages in modified EDTPA with mini-peeling of dura propria
Removal of the ACP is one of the essential skull base techniques to treat skull base tumors. Cadaveric studies have shown that the anterior clinoidectomy technique can double the exposure and mobilization of the optic nerve and ICA, as well as triple or quadruple the opticocarotid triangle width and oculomotor triangle size.[
Bleeding from the CS during trans-CS approaches is one of the drawbacks. The opened Dolenc's triangle and Mullan's triangle between V1 and V2 were major bleeding points during the epidural procedures in our modified EDTPA but the bleeding could be safely controlled by either Surgicel (Johnson and Johnson's Ethicon subsidiary) packing or fibrin glue direct injection without any resultant symptoms.[
Surgical advantages of modified EDTPA with mini-peeling of dura propria for removal of paraclinoid and/or parasellar tumors
It is generally recognized that safe removal of the tumors located in the paraclinoid and/or parasellar areas is difficult because the tumor may engulf neurovascular structures such as the optic apparatus and major cerebral arteries including their perforators. Moreover, some tumors extend into the CS, optic canal, or infraoptic and subchiasmatic regions.
Our modified EDTPA with mini-peeling of dura propria requires dura propria peeling of the medial temporal lobe dura as well as periosteal dissection of the both frontal base dura mater and temporal base dura mater. The ACP is epidurally removed. During these epidural procedures, the meningeal blood supply of the tumors is automatically and efficiently diminished. The modified EDTPA approach also allows extradural early localization and exposure of the optic nerve. The exposed optic nerve can be followed from the optic canal proximally toward any tumor in an intradural location, which allows the surgeon to recognize the exact location of the optic nerve early in the surgical procedure. In addition, the modified EDTPA facilitates access to such difficult locations, especially in the presence of tumor extension into the CS, optic canal, or infraoptic and subchiasmatic regions. The removed ACP gives us enough working space in the paraclinoid area. Furthermore, incision of the falciform ligament and partial incision of the distal dural ring facilitates mobilization of the optic nerve and ICA, which can help to widen the opticocarotid space and oculomotor trigone to access and effectively remove tumor in the retrocarotid and infrachiasmatic space with minimum chances of injuring important neurovascular structures. Indeed, the safe mobilization of optic nerve and ICA is one of the most important advantages of the modified EDTPA. Consequently, the EDTPA technique was useful for maximal resection of tumors involving the retrochiasmatic space with relatively low incidence of postoperative visual disturbance. In all our patients, the pituitary stalks were preserved, so no patient required hormonal replacement or suffered hypothalamic dysfunction postoperatively, and patients with tumors extending into the optic canal achieved improvement in visual deterioration. Oculomotor nerve palsy occurred in only one patient but completely recovered within 3 months. Before drilling, the inferolateral part of the ACP should be dissected because the extradural part of the oculomotor nerve passes there. In our case, oculomotor paresis could be due to the detachment and movement of the intradural part, which was not related to the surgical approach. The medial tentorium incision and temporal lobe retraction over the dura mater gave us enough space in the pretemporal area to access the tumor widely from the antero-lateral trajectory. Due to the temporal lobe retraction over the dura mater, we did not experience any case of temporal lobe contusion.
CONCLUSION
A series of 27 patients with paraclinoid and/or parasellar tumors who underwent modified EDTPA with mini-peeling of the dura propria was analyzed. Total or subtotal resection of the tumors was achieved with minimal surgical complications in majority of the patients. The modified EDTPA technique leads to excellent improvement of the visual function and overall clinical outcomes in patients with tumors encasing the optic nerve and ICA or extending into the optic canal and infrachiasmatic space. This modified approach provides safe tumor removal with less invasive trans-cavernous approach.
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Coscarella E, Bakaya MK, Morcos JJ. An alternative extradural exposure to the anterior clinoid process: The superior orbital fissure as a surgical corridor. Neurosurgery. 2003. 53: 162-6
2. Day JD, Giannotta SL, Fukushima T. Extradural temporopolar approach to lesions of the upper basilar artery and infrachiasmatic region. J Neurosurg. 1994. 81: 230-5
3. Day JD, Fukushima T, Giannotta SL. Cranial base approaches to posterior circulation aneurysms. J Neurosurg. 1997. 87: 544-54
4. Dolenc VV. Direct microsurgical repair of intracavernous vascular lesions. J Neurosurg. 1983. 58: 824-31
5. Dolenc VV. A combined epi- and subdural direct approach to carotid-ophthalmic artery aneurysms. J Neurosurg. 1985. 62: 667-72
6. Dolenc VV, Skrap M, Sustersic J, Skrbec M, Morina A. A transcavernous-transsellar approach to the basilar tip aneurysms. Br J Neurosurg. 1987. 1: 251-9
7. Dolenc VV. Transcranial epidural approach to pituitary tumors extending beyond the sella. Neurosurgery. 1997. 41: 542-52
8. Evans JJ, Hwang YS, Lee JH. Pre- versus post-anterior clinoidectomy measurements of the optic nerve, internal carotid artery and opticocarotid triangle. A cadaveric morphometric study. Neurosurgery. 2000. 46: 1018-23
9. Kadis GN, Mount LA, Ganti SR. The importance of early diagnosis and treatment of the meningiomas of the planum sphenoidale and tuberculum sellae: A retrospective study of 105 cases. Surg Neurol. 1979. 12: 367-71
10. Lee JH, Jeun SS, Evans J, Kosmorsky G. Surgical management of clinoidal meningiomas. Neurosurgery. 2001. 48: 1012-21
11. Mori K, Yamamoto T, Oyama K, Ueno H, Nakao Y, Honma K. Modified three- dimensional skull base model with artificial dura mater, cranial nerves, and venous sinuses for training in skull base surgery: Technical note. Neurol Med Chir (Tokyo). 2008. 48: 582-7
12. Mori K. Dissectable modified three-dimensional temporal bone and whole skull base models for training in skull base approaches. Skull Base. 2009. 19: 333-44
13. Mori K, Yamamoto T, Oyama K, Nakao Y. Modification of three-dimensional prototype temporal bone model for training in skull-base surgery. Neurosurg Rev. 2009. 32: 233-8
14. Noguchi A, Balasingam V, Shiokawa Y, McMenomey SO, Delashaw JB. Extradural anterior clinoidectomy. Technical note. J Neurosurg. 2005. 102: 945-50
15. Otani N, Muroi C, Yano H, Khan N, Pangalu A, Yonekawa Y. Surgical management of tuberculum sellae meningioma: Role of of selective extradural anterior clinoidectomy. Br J Neurosurg. 2006. 20: 129-38
16. Otani N, Wada K, Toyooka T, Fujii K, Kobayashi Y, Mori K. Operative surgical nuances of modified extradural temporopolar approach with mini-peeling of dura propria based on cadaveric anatomical study of lateral cavernous structures. Surgical Neurol Int. 2016. 7: S454-8
17. Sade B, Kweon CY, Evans JJ, Lee JH. Enhanced exposure of caroticooculomotor triangle following extradural anterior clinoidectomy: A comparative anatomical study. Skull Base. 2005. 15: 157-61
18. Symon L, Rosenstein J. Surgical management of suprasellar meningioma. Part 1: The influence of tumor size, duration of symptoms and microsurgery on surgical outcome in 101 consecutive cases. J Neurosurg. 1984. 61: 633-41
19. Toyooka T, Otani N, Wada K, Tomiyama A, Ueno H, Fujii K. Effect of fibrin glue injection into the cavernous sinus for hemostasis during transcavernous surgery on the cerebral venous draining system. Oper Neurosurg. 2017. 13: 224-31
20. Yonekawa Y, Ogata N, Imhof HG, Olivecrona M, Strommer K, Kwak TE. Selective extradural anterior clinoidectomy for supra- and parasellar processes. Technical note. J Neurosurg. 1997. 87: 636-42
21. Yoon BH, Kim HK, Park MS, Kim SM, Chung SY, Lanzino G. Meningeal layers around anterior clinoid process as a delicate area in extradural anterior clinoidectomy: Anatomical and clinical study. J Korean Neurosurg Soc. 2012. 52: 391-5