- Department of Neurosurgery, Neuroscience Institute, Henry Ford Hospital, 2799 W Grand Blvd, Detroit, Michigan, USA
- Department of Radiation Oncology, Neuroscience Institute, Henry Ford Hospital, 2799 W Grand Blvd, Detroit, Michigan, USA
- Department of Radiology, Neuroscience Institute, Henry Ford Hospital, 2799 W Grand Blvd, Detroit, Michigan, USA
Correspondence Address:
Victor Chang
Department of Neurosurgery, Neuroscience Institute, Henry Ford Hospital, 2799 W Grand Blvd, Detroit, Michigan, USA
DOI:10.4103/sni.sni_383_17
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Hesham Mostafa Zakaria, Erinma Elibe, Mohamed Macki, Richard Smith, David Boyce-Fappiano, Ian Lee, Brent Griffith, Farzan Siddiqui, Victor Chang. Morphometrics predicts overall survival in patients with multiple myeloma spine metastasis: A retrospective cohort study. 22-Aug-2018;9:172
How to cite this URL: Hesham Mostafa Zakaria, Erinma Elibe, Mohamed Macki, Richard Smith, David Boyce-Fappiano, Ian Lee, Brent Griffith, Farzan Siddiqui, Victor Chang. Morphometrics predicts overall survival in patients with multiple myeloma spine metastasis: A retrospective cohort study. 22-Aug-2018;9:172. Available from: http://surgicalneurologyint.com/surgicalint-articles/morphometrics-predicts-overall-survival-in-patients-with-multiple-myeloma-spine-metastasis-a-retrospective-cohort-study/
Abstract
Background:Treatment strategies for spinal metastases for myeloma range from conservative measures (radiation and chemotherapy) to invasive (surgical). Identifying better predictors of overall survival (OS) would help in surgical decision making. Analytic morphometrics has been shown to predict survival in oncologic patients, and our study evaluates whether morphometrics is predictive of survival in patients with multiple myeloma (MM) spinal metastases.
Methods:For this observational retrospective cohort study, we identified 46 patients with MM spinal metastases who had undergone stereotactic body radiation therapy. OS was the primary outcome measure. Morphometric analysis of the psoas muscle was performed using computed tomography scans of the lumbar spine.
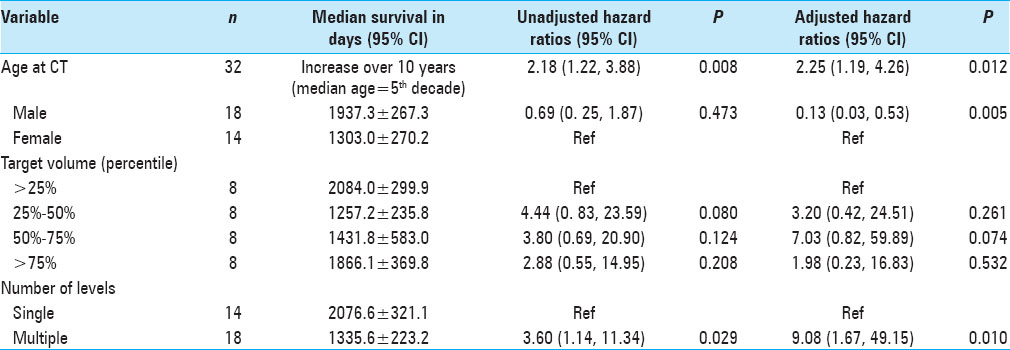
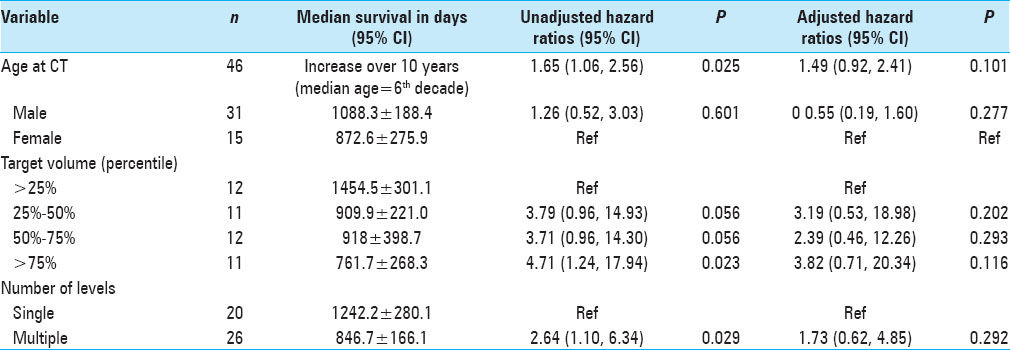
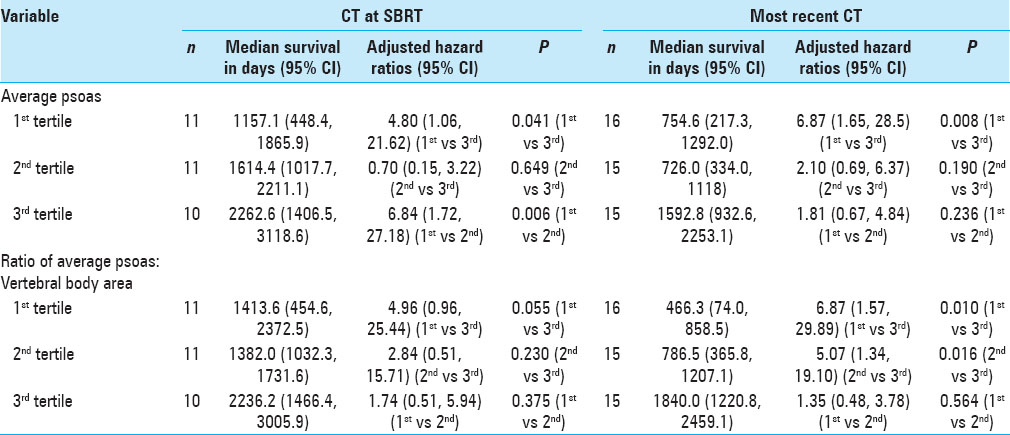
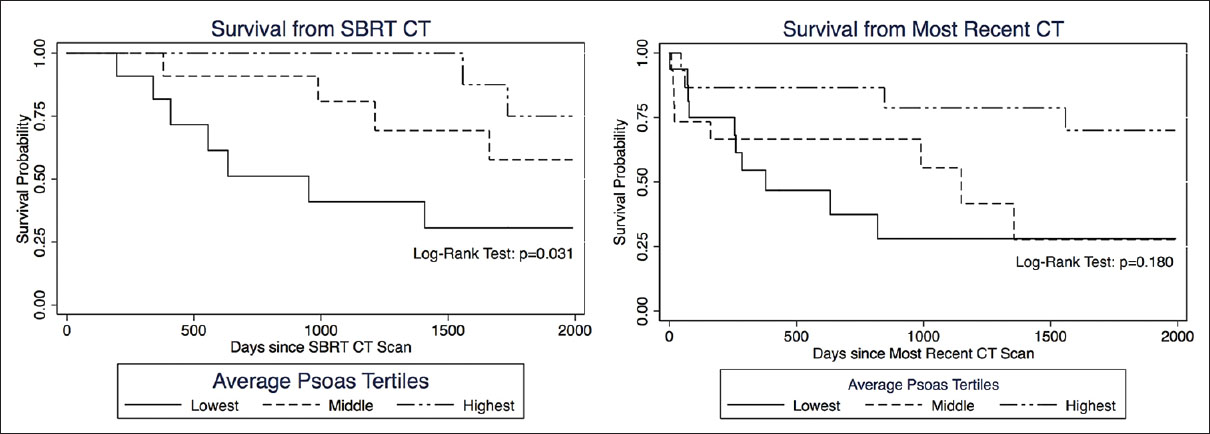
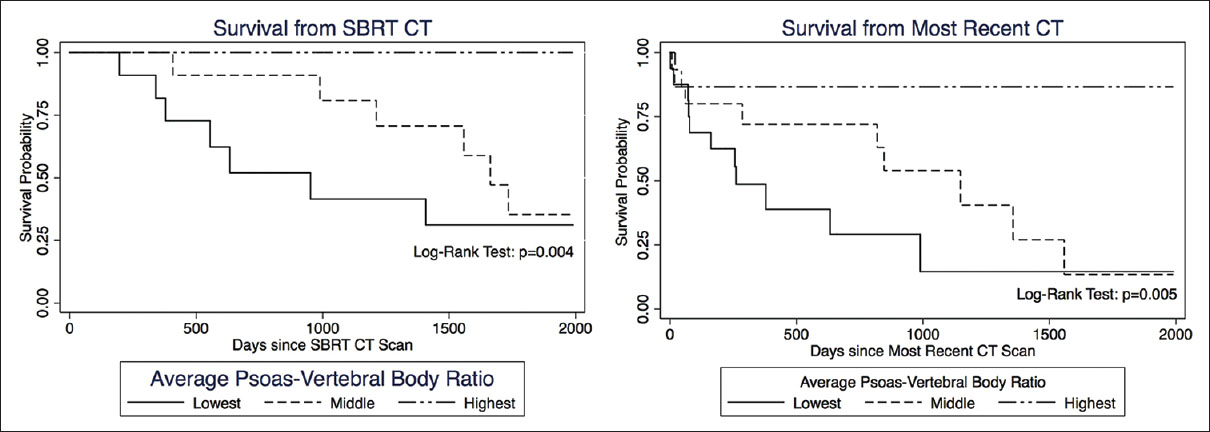
Results:OS was statistically correlated with age (P = 0.025), tumor burden (P = 0.023), and number of levels radiated (P = 0.029), but not with gender. Patients in the lowest tertile of average psoas size had significantly shorter survival compared to the highest tertile, hazard ratio (HZ) 6.87 (95% CI = 1.65–28.5, P = 0.008). When calculating the psoas size to vertebral body ratio and correlating this measure to OS, the lowest tertile again had significantly shorter OS compared to the highest tertile, HZ 6.87 (95% CI = 1.57–29.89, P = 0.010); the middle tertile also showed significantly shorter OS compared to the highest tertile, HZ 5.07 (95% CI = 1.34–19.10, P = 0.016). Kaplan–Meier survival curves were used to visually illustrate the differences in survival between different tertiles (Log-rank test P = 0.006).
Conclusions:Morphometric analysis successfully predicts long-term survival in patients with MM. More research is needed to validate these results and to see if these methodologies can be applied to other cancer histologies.
Keywords: Frailty index, morphometric analysis, multiple myeloma mortality, oncologic outcomes, spinal metastases
INTRODUCTION
Multiple myeloma (MM) is a malignancy defined by the clonal proliferation of monoclonal plasma cells producing M-protein and ultimately leads to end organ disease including hypercalcemia, renal insufficiency, anemia, and bone metastases.[
A hallmark of human senescence is frailty, which is defined as a “decreased reserve to physiologic stressors.”[
In this study, we applied morphometric analysis of psoas size to predict OS in patients with MM metastases to the spine. We hypothesized that sarcopenic patients, as measured by cross-sectional psoas size area, would have shorter OS.
MATERIALS AND METHODS
This study was approved by our Institutional Review Board (IRB #9813). We accessed a registry of spinal metastases patients for the period from 2002 to 2012 who had undergone stereotactic body radiation therapy (SBRT) at our institution, and among these, we identified all patients who had a primary diagnosis of MM, as described by pathology reports or clinical records. Of these patients, we identified patients with myeloma spine disease that had a computed tomography (CT) imaging scan within 6 months of SBRT, or identified the most recent CT scan after completion of radiation.
Data sources and variables
Electronic medical records of these patients were the primary data source reviewed. Using previously described methodology,[
Radiation treatment protocols
At our institution, the gross tumor volume, the clinical target volume, and the planning target volume for a given patient are all identical; during SRS treatment planning, no expansion margin was added to the gross tumor, and thus, the gross tumor volume was equal to the planning target volume. Margins were created using T2-weighted magnetic resonance images to delineate the spinal cord 6 mm above and 6 mm below the defined gross tumor volume. The entire affected vertebral body was included in the target volume. All doses were administered in a single fraction. While EBRT is typically used for MM spinal metastases at other institutions, all the patients in our cohort were carefully selected to receive SBRT/SRS. Each patient was discussed in a multidisciplinary spine tumor board attended by radiation oncologists, neurosurgeons, neuroradiologists, and medical oncologists. Recommendations regarding whether a patient was a suitable candidate for spine SRS were made based on consensus opinion.
Statistical analysis
Measurements of the psoas muscle size were divided into tertiles according to the value of the psoas area encountered. To account for patient stature, the ratio of average psoas size to that patient's vertebral body cross-sectional area at L4 was considered. The rationale here is that the sizes of these two structures should be proportional to each other and independent of patient stature because the psoas originates from the vertebral body; a large and a small person would have a large and small psoas and vertebral body, respectively, but the psoas/vertebral body ratio should be consistent for both patients.[
Subsequently, gross tumor volume for SBRT reflects the cumulative tumor volume in the spinal column, and thus, serves as a surrogate marker of disease burden; this hypothesis was tested by dividing the measurements of target volumes into quartiles and subsequently calculating the hazards of death within each quartile. We hypothesize that there was a correlation between the hazards of death and increasing tumor volume (i.e., the hazard ratio would increase with increasing tumor volume).
The main outcome of interest was OS, which was measured from the date of the patient's selected CT scan to the date of death or last follow-up. We chose to measure OS from the date of CT scan and not from the date of diagnosis of myeloma or other time point for a number of reasons. Most importantly, sarcopenia is a time dependent process; we have observed that during the course of oncologic disease muscle mass can change, such as when patients are undergoing chemotherapy. Therefore, measuring death from a time point other than the date of CT would be confounding as patient frailty and sarcopenia are not measured accurately at that time point. Moreover, due to the retrospective nature of this study, not many patients will have a CT scan showing the psoas muscle at the time of diagnosis of myeloma or even from the diagnosis of spinal disease. Therefore, to guarantee a consistent point in time and disease as well as a large patient sample, it was most logical to start from the date of the CT scan. The CT scan chosen was either at the time of SBRT or the most recent CT scan on record. Patients who did not have a known date of death were censored to the date of last follow-up. We are unable to determine cause of death for most patients due to incomplete records.
The median OS in days along with the corresponding 95% confidence interval (CI) was computed for all patients, as well as for subsets of interest. Cox proportional hazards regression analyses provided an estimate of the hazard ratios (HRs) of death. Unadjusted HR tested for the likelihood of mortality with any given variable of interest. Multivariate analysis of adjusted HR controlled for clinically relevant predictors of death along with statistically significant predictors in the univariate analysis. Kaplan–Meier estimates of time to death were categorized by average psoas tertile. The log-rank test was used to assess differences among the three Kaplan–Meier curves (i.e., tertiles). The Kaplan–Meier curves were censored at 2000 days after imaging, which roughly correlates to 5-year mortality. Patients followed beyond 5 years were considered to be without active systemic disease; thus, changes in psoas size secondary to muscle remodeling no longer correlated with psoas measurements at the time of CT scan. All testing was done at a statistical significance level of 0.05. All statistical analyses were performed using STATA (version 13.0, College Station, TX, USA) and Microsoft Excel.
RESULTS
Participants and descriptive data
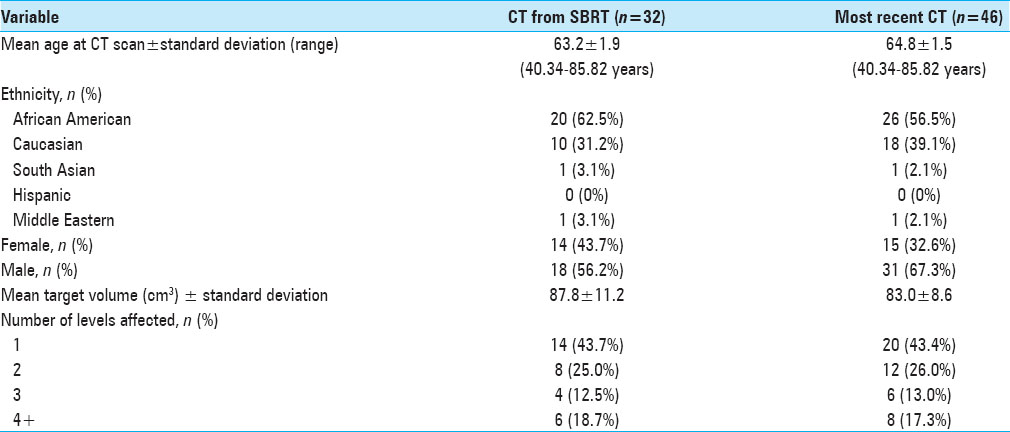
Of the patients with MM spine disease within our database, we identified 32 patients who had a CT scan showing the psoas muscle within 6 months of SBRT. We took measurements from the most recent and available CT scan of 46 patients. The mean age of the SBRT cohort was 63.2 (SD ± 1.9) and for most recent CT cohort was 64.8 years (SD ± 1.5yrs) [
The mean OS and standard deviation for the SBRT cohort of 32 patients was 1659.8 days (range, 196–4577), and for the 46 recent CT patients it was 1018.6 days (range, 1–4577 days).
When calculating the psoas size to vertebral body size ratio [
DISCUSSION
Our study results show that morphometric analysis of psoas size can be used as a suitable predictor of OS in select patients with metastatic spine disease from MM. This validates our previously published work on the reliability of psoas size in predicting OS in patients with lung cancer metastases to the spine.[
Limitations
Our study is limited by its retrospective nature and the fact that data were acquired in a single institution. We are also limited by the electronic medical records, which may have intrinsic and hidden bias caused by patient selection for intent to treat. Our patient population also only includes those who were referred to our center and also successfully underwent SBRT for their metastatic spinal disease, thereby excluding other patients in earlier stages of disease or those who did not qualify to undergo this specific type of radiation. However, given the standard practice at our institution, we believe that the latter is a small number of patients and that our cohort is representative of the population. We are limited by patient sample size as well. Some of our results approached significance, and during preliminary analysis, we noted that as we increased the size of our patient cohort, our results became significant. Prospective multicenter studies are needed to validate our findings. If they are validated, morphometrics could be used to assist in patient selection surgery and for tailoring specific oncologic treatment regimens.
Interpretation
This study has potential implications for decision making in neurosurgery and oncology. While MM commonly metastasizes to the spine, its current tumor staging system is independent of the extent of osseous involvement;[
Spine surgery in this population has been shown to have the potential to improve patient outcomes,[
Any surgery carries inherent risks, and a postoperative morbidity may debilitate the patient and hasten demise. Therefore, an objective process to assess fitness for surgery and/or longevity is beneficial, as it establishes a basis for therapeutic decision making. Morphometrics is a novel, objective, and comparably simple method to obtain a surrogate measure that allows one to assess an individual's overall health for surgery, as evidenced by its ability to predict outcomes and overall mortality[
In our study, the use of the psoas size to vertebral body ratio significantly strengthened prognostication. However, it is intuitive that patients who have smaller stature and therefore objectively smaller musculature are not necessarily more frail. By normalizing the psoas muscle to the size of the vertebral body we could also account for stature and increase the sensitivity of our analysis; the middle tertile being at higher risk for death than the highest tertile (P = 0.016), and the log-rank test for the Kaplan–Meier survival curves reached statistical significance (P = 0.006). Evidence suggests that the psoas/vertebral body ratio may be more suitable in male patients,[
Generalizability
This study is likely generalizable to all patients with MM with metastases to the spinal column. This study population is representative of patients who were referred to radiation treatment of spinal metastases regardless of the decision for surgery, and hence, is not limited by operative plan.
CONCLUSION
Morphometric analysis of psoas size can be used in predicting survival in select patients with MM metastases to the spine. This information can assist in patient selection for surgery, as well as to tailor oncologic treatments.
Disclosures
Ian Lee: Consulting agreement with Medtronic; Honoraria from Varian Medical Systems; Consulting agreement from Monteris.
Farzan Siddiqui: Grants from Varian Medical Systems; Honoraria from MD Anderson SBRT Symposium; Honoraria from St. John Providence Hospital; Honoraria from American College of Veterinary Radiology; Medical grants from Philips Medical; Honoraria from Wayne State University.
Victor Chang: Consulting agreement with DepuySynthes; Consulting agreement with Globus; Research support from Medtronic.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Amelot A, Cristini J, Salaud C, Moles A, Hamel O, Moreau P. Overall survival in spine myeloma metastases: Difficulties in predicting with prognostic scores. Spine. 2017. 42: 400-6
2. Amelot A, Moles A, Cristini J, Salaud C, Touzeau C, Hamel O. Predictors of survival in patients with surgical spine multiple myeloma metastases. Surg Oncol. 2016. 25: 178-83
3. Amrock LG, Deiner S. The implication of frailty on preoperative risk assessment. Curr Opin Anaesthesiol. 2014. 27: 330-5
4. Boland E, Eiser C, Ezaydi Y, Greenfield DM, Ahmedzai SH, Snowden JA. Living with advanced but stable multiple myeloma: A study of the symptom burden and cumulative effects of disease and intensive (hematopoietic stem cell transplant-based) treatment on health-related quality of life. J Pain Symptom Manage. 2013. 46: 671-80
5. Bollen L, de Ruiter GC, Pondaag W, Arts MP, Fiocco M, Hazen TJ. Risk factors for survival of 106 surgically treated patients with symptomatic spinal epidural metastases. Eur Spine J. 2013. 22: 1408-16
6. Choi D, Crockard A, Bunger C, Harms J, Kawahara N, Mazel C. Review of metastatic spine tumour classification and indications for surgery: The consensus statement of the Global Spine Tumour Study Group. Eur Spine J. 2010. 19: 215-22
7. Choi D, Fox Z, Albert T, Arts M, Balabaud L, Bunger C. Rapid improvements in pain and quality of life are sustained after surgery for spinal metastases in a large prospective cohort. Br J Neurosurg. 2016. 30: 337-44
8. Cloney M, D’Amico R, Lebovic J, Nazarian M, Zacharia BE, Sisti MB. Frailty in geriatric glioblastoma patients: A predictor of operative morbidity and outcome. World Neurosurg. 2016. 89: 362-7
9. Cocks K, Cohen D, Wisloff F, Sezer O, Lee S, Hippe E. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-MY20) in assessing the quality of life of patients with multiple myeloma. Eur J Cancer. 2007. 43: 1670-8
10. Coleman RE. Skeletal complications of malignancy. Cancer. 1997. 80: 1588-94
11. Croucher PI, Apperley JF. Bone disease in multiple myeloma. Br J Haematol. 1998. 103: 902-10
12. Dea N, Versteeg A, Fisher C, Kelly A, Hartig D, Boyd M. Adverse events in emergency oncological spine surgery: A prospective analysis. J Neurosurg Spine. 2014. 21: 698-703
13. Durr HR, Wegener B, Krodel A, Muller PE, Jansson V, Refior HJ. Multiple myeloma: surgery of the spine: Retrospective analysis of 27 patients. Spine. 2002. 27: 320-4
14. Ebbeling L, Grabo DJ, Shashaty M, Dua R, Sonnad SS, Sims CA. Psoas: lumbar vertebra index: Central sarcopenia independently predicts morbidity in elderly trauma patients. Eur J Trauma Emerg Surg. 2014. 40: 57-65
15. Englesbe MJ, Lee JS, He K, Fan L, Schaubel DE, Sheetz KH. Analytic morphomics, core muscle size, and surgical outcomes. Ann Surg. 2012. 256: 255-61
16. Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C. Sarcopenia and mortality after liver transplantation. J Am Coll Surgeons. 2010. 211: 271-8
17. Fourney DR, Frangou EM, Ryken TC, Dipaola CP, Shaffrey CI, Berven SH. Spinal instability neoplastic score: An analysis of reliability and validity from the spine oncology study group. J Clin Oncol. 2011. 29: 3072-7
18. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J. Frailty in older adults: Evidence for a phenotype. J Gerontol A, Biol Sci Med Sci. 2001. 56: M146-6
19. Ghori AK, Leonard DA, Schoenfeld AJ, Saadat E, Scott N, Ferrone ML. Modeling 1-year survival after surgery on the metastatic spine. Spine J. 2015. 15: 2345-50
20. Guzik G. Oncological and functional results of the surgical treatment of vertebral metastases in patients with multiple myeloma. BMC Surg. 2017. 17: 92-
21. Hasselager R, Gogenur I. Core muscle size assessed by perioperative abdominal CT scan is related to mortality, postoperative complications, and hospitalization after major abdominal surgery: A systematic review. Langenbeck's Arch Surg. 2014. 399: 287-95
22. Holman PJ, Suki D, McCutcheon I, Wolinsky JP, Rhines LD, Gokaslan ZL. Surgical management of metastatic disease of the lumbar spine: Experience with 139 patients. J Neurosurg Spine. 2005. 2: 550-63
23. Itoh S, Shirabe K, Matsumoto Y, Yoshiya S, Muto J, Harimoto N. Effect of body composition on outcomes after hepatic resection for hepatocellular carcinoma. Ann Surg Oncol. 2014. 21: 3063-8
24. Janssen SJ, Teunis T, van Dijk E, Ferrone ML, Shin JH, Hornicek F. Validation of the Spine Oncology Study Group-Outcomes Questionnaire to assess quality of life in patients with metastatic spine disease. Spine J. 2017. 17: 768-76
25. Kariyawasan CC, Hughes DA, Jayatillake MM, Mehta AB. Multiple myeloma: Causes and consequences of delay in diagnosis. QJM. 2007. 100: 635-40
26. Kim CH, Chung CK, Jahng TA, Kim HJ. Resumption of ambulatory status after surgery for nonambulatory patients with epidural spinal metastasis. Spine J. 2011. 11: 1015-23
27. Kim JM, Losina E, Bono CM, Schoenfeld AJ, Collins JE, Katz JN. Clinical outcome of metastatic spinal cord compression treated with surgical excision +/- radiation versus radiation therapy alone: A systematic review of literature. Spine. 2012. 37: 78-84
28. Kumar SK, Callander NS, Alsina M, Atanackovic D, Biermann JS, Chandler JC. Multiple Myeloma, Version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017. 15: 230-69
29. Kuroki LM, Mangano M, Allsworth JE, Menias CO, Massad LS, Powell MA. Pre-operative assessment of muscle mass to predict surgical complications and prognosis in patients with endometrial cancer. Ann Surg Oncol. 2015. 22: 972-9
30. Laufer I, Rubin DG, Lis E, Cox BW, Stubblefield MD, Yamada Y. The NOMS framework: Approach to the treatment of spinal metastatic tumors. Oncologist. 2013. 18: 744-51
31. Lee BH, Park JO, Kim HS, Park YC, Lee HM, Moon SH. Perioperative complication and surgical outcome in patients with spine metastases: Retrospective 200-case series in a single institute. Clin Neurol Neurosurg. 2014. 122: 80-6
32. Lee JS, He K, Harbaugh CM, Schaubel DE, Sonnenday CJ, Wang SC. Frailty, core muscle size, and mortality in patients undergoing open abdominal aortic aneurysm repair. J Vasc Surg. 2011. 53: 912-7
33. Leithner A, Radl R, Gruber G, Hochegger M, Leithner K, Welkerling H. Predictive value of seven preoperative prognostic scoring systems for spinal metastases. Eur Spine J. 2008. 17: 1488-95
34. Majeed H, Kumar S, Bommireddy R, Klezl Z, Calthorpe D. Accuracy of prognostic scores in decision making and predicting outcomes in metastatic spine disease. Ann R Coll Surg Engl. 2012. 94: 28-33
35. Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010. 210: 901-8
36. Miller AL, Min LC, Diehl KM, Cron DC, Chan CL, Sheetz KH. Analytic morphomics corresponds to functional status in older patients. J Surg Res. 2014. 192: 19-26
37. Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J. Sarcopenia is a negative prognostic factor after curative resection of colorectal cancer. Ann Surg Oncol. 2015. 22: 2663-8
38. Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet. 2005. 366: 643-8
39. Peng P, Hyder O, Firoozmand A, Kneuertz P, Schulick RD, Huang D. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg. 2012. 16: 1478-86
40. Phillips A, Strobl R, Vogt S, Ladwig KH, Thorand B, Grill E. Sarcopenia is associated with disability status-results from the KORA-Age study. Osteoporos Int. 2017. 28: 2069-79
41. Quraishi NA, Rajabian A, Spencer A, Arealis G, Mehdian H, Boszczyk BM. Reoperation rates in the surgical treatment of spinal metastases. Spine J. 2015. 15: S37-43
42. Ruiz M, Cefalu C, Reske T. Frailty syndrome in geriatric medicine. Am J Med Sci. 2012. 344: 395-8
43. Sheetz KH, Zhao L, Holcombe SA, Wang SC, Reddy RM, Lin J. Decreased core muscle size is associated with worse patient survival following esophagectomy for cancer. Dis Esophagus. 2013. 26: 716-22
44. Sonmez M, Akagun T, Topbas M, Cobanoglu U, Sonmez B, Yilmaz M. Effect of pathologic fractures on survival in multiple myeloma patients: A case control study. J Exp Clin Cancer Res. 2008. 27: 11-
45. Swanson S, Patterson RB. The correlation between the psoas muscle/vertebral body ratio and the severity of peripheral artery disease. Ann Vasc Surg. 2015. 29: 520-5
46. Tatsui CE, Suki D, Rao G, Kim SS, Salaskar A, Hatiboglu MA. Factors affecting survival in 267 consecutive patients undergoing surgery for spinal metastasis from renal cell carcinoma. J Neurosurg Spine. 2014. 20: 108-16
47. Terpos E, Mihou D, Szydlo R, Tsimirika K, Karkantaris C, Politou M. The combination of intermediate doses of thalidomide with dexamethasone is an effective treatment for patients with refractory/relapsed multiple myeloma and normalizes abnormal bone remodeling, through the reduction of sRANKL/osteoprotegerin ratio. Leukemia. 2005. 19: 1969-76
48. Terpos E, Politou M, Rahemtulla A. New insights into the pathophysiology and management of bone disease in multiple myeloma. Br J Haematol. 2003. 123: 758-769
49. Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine. 2005. 30: 2186-91
50. Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine. 2001. 26: 298-306
51. Vogel CL, Yanagihara RH, Wood AJ, Schnell FM, Henderson C, Kaplan BH. Safety and pain palliation of zoledronic acid in patients with breast cancer, prostate cancer, or multiple myeloma who previously received bisphosphonate therapy. Oncologist. 2004. 9: 687-95
52. Waits SA, Kim EK, Terjimanian MN, Tishberg LM, Harbaugh CM, Sheetz KH. Morphometric age and mortality after liver transplant. JAMA Surg. 2014. 149: 335-40
53. Zadnik PL, Goodwin CR, Karami KJ, Mehta AI, Amin AG, Groves ML. Outcomes following surgical intervention for impending and gross instability caused by multiple myeloma in the spinal column. J Neurosurg Spine. 2015. 22: 301-9
54. Zakaria HM, Basheer A, Boyce-Fappiano D, Elibe E, Schultz L, Lee I. Application of morphometric analysis to patients with lung cancer metastasis to the spine: A clinical study. Neurosurg Focus. 2016. 41: E12-
55. Zakaria HM, Basheer A, Boyce-Fappiano D, Elibe E, Schultz L, Lee I.editors. Application of morphometrics as a predictor for survival in patients with prostate cancer metastasis to the spine [abstract]. Joint Spine Section Annual Meeting. 2017. p.
56. Zakaria HM, Schultz L, Mossa-Basha F, Griffith B, Chang V. Morphometrics as a predictor of perioperative morbidity after lumbar spine surgery. Neurosurg Focus. 2015. 39: E5-