- Department of Experimental Biomedicine and Clinical Neurosciences, School of Medicine, Neurosurgical Clinic, University of Palermo, Palermo, Italy
Correspondence Address:
Rosario Maugeri
Department of Experimental Biomedicine and Clinical Neurosciences, School of Medicine, Neurosurgical Clinic, University of Palermo, Palermo, Italy
DOI:10.4103/sni.sni_68_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Rosario Maugeri, Roberto G. Giammalva, Francesca Graziano, Luigi Basile, Carlo Gulì, Antonella Giugno, Domenico G. Iacopino. Never say never again: A bone graft infection due to a hornet sting, thirty-nine years after cranioplasty. 10-Aug-2017;8:189
How to cite this URL: Rosario Maugeri, Roberto G. Giammalva, Francesca Graziano, Luigi Basile, Carlo Gulì, Antonella Giugno, Domenico G. Iacopino. Never say never again: A bone graft infection due to a hornet sting, thirty-nine years after cranioplasty. 10-Aug-2017;8:189. Available from: http://surgicalneurologyint.com/surgicalint-articles/never-say-never-again-a-bone-graft-infection-due-to-a-hornet-sting-thirty%e2%80%91nine-years-after-cranioplasty/
Abstract
Background:Cranioplasty (CP) is a widespread surgical procedure aimed to restore skull integrity and physiological cerebral hemodynamics, to improve neurological functions and to protect the underlying brain after a life-saving decompressive craniectomy (DC). Nevertheless, CP is still burdened by surgical complications, among which early or late graft infections are the most common outcome-threatening ones.
Case Description:We report the case of 48-year-old man admitted to our neurosurgical unit because of a painful right frontal swelling and 1-week purulent discharge from a cutaneous fistula. He had been undergone frontal CP because of severe traumatic brain injury (TBI) when he was 9-year-old. Since then, his medical history has been being unremarkable without any surgical or infective complication of the graft for 39 years, until he was accidentally stung by a hornet in the frontal region. After the CT scan and laboratory findings had evidenced a probable infection of the graft, the patient was treated by vancomycin and cefepime before he underwent surgical revision of its former CP, with the removal of the graft and the debridement of the surgical field. Subsequent bacteriological tests revealed Staphylococcus aureus as causal agent of that infection.
Conclusion:This case illustrates an anecdotal example of very late CP infection, due to an unpredictable accident. Due to lack of consensus on risk factors and on conservative or surgical strategy in case of graft infection, we aimed to share our surgical experience.
Keywords: Cranioplasty, late infection management, risk factors, surgical complications
INTRODUCTION
Decompressive craniectomy (DC) has become a widespread procedure for treating life-threatening conditions that lead to a higher intracranial pressure (ICP).[
Nevertheless, severe traumatic brain injury (TBI) still remains the most common indication for DC in many clinical series.[
CASE DESCRIPTION
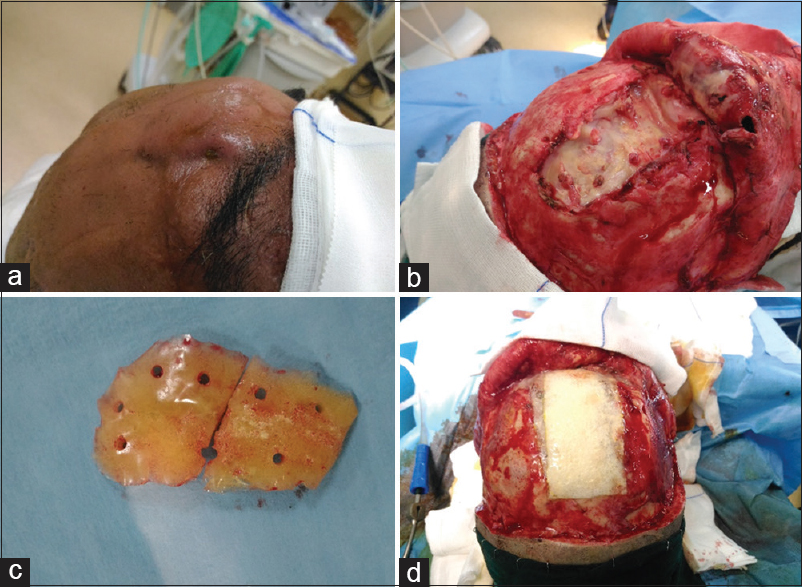
A 48-year-old male was admitted to our neurosurgical unit because of a frontal swelling with purulent discharge from a cutaneous fistula. In his clinical history, he reported a previous frontal TBI when he was 9-year-old. For this reason, he had undergone a frontal craniectomy with subsequent unspecified CP. Since that, his clinical history has been unremarkable for 39 years, without any surgical or infective complications related to the cranial surgical procedure. A week before his admission he had been stung by a hornet while he was working in the countryside, with a progressive swelling of frontal soft tissues, temperature raising, and local pain with inflammatory state. During that week, a progressive purulent discharge has onset from the site of the sting, so he referred to the emergency department. At the admission, he was awake and aware [Glasgow Coma Scale 15 (GSC 15)], his temperature was 37.8°C and his neurological examination was negative. Initial laboratory studies revealed a pathological increased value of white blood cell (WBC) count (22 × 109/L with 82% neutrophils, 14% lymphocytes, and 7% monocytes) and of serum C-reactive protein level (57 mg/dL with a normal range 0.08–1.5 mg/dL). A head CT scan revealed an irregular subcutaneous fluid collection, adherent to the graft's anterior face, a peripheral soft tissues edema, and a frontal hypodensity in brain parenchyma contiguous to the graft. The administration of contrast medium revealed a moderate peripheral enhancement of the collection. Because of the radiological evidence of the graft infection with subcutaneous abscess, the patient was then transferred to our neurosurgical unit. A swab test of the purulent discharge from the cutaneous fistula was performed, and, after a multidisciplinary consult, a polychemotherapy was started (1 g of vancomycin and 2 g of cefepime every 12 h). Thirty-six hours after his admission, the patient underwent surgical toilet and removal of the graft. This appeared as a porous acrylic graft, with several fibrotic bands firmly tied with the skin and the underlying synthetic dural substitute. All the samples were harvested for microbiological exam, while the surgical site was washed with iodine solution, peroxide, isotonic saline, and rifampicine. At the end, a spongy layer of dural substitute with two overlaying patch of fibrin sealant (Tachosil©) and some fibrin glue (Vivostat©) was then used to ensure dural seal and to contribute in protecting the brain parenchyma under the bone defect [Figure
Nor titanium mesh or other allograft was applied on the former craniotomy in order to prevent the rejection, waiting for the resolution of the infection in analogy with other surgical procedures.[
Further bacteriological tests revealed Staphylococcus aureus as causal agent of that graft infection. Due to this, the patient was discharged 7 days after surgical procedure under an antibiotic therapy with linezolid and ceftazidime and he is still in follow-up for further CP after the infection resolution and inflammatory markers normalization.
DISCUSSION
CP is a common surgical procedure requisite to restore skull integrity in such cases where it has been compromised. Moreover, it has been demonstrated that CP can be effective in preventing seizures or cerebral atrophy after a DC so avoiding the “trephined syndrome,”[
CP failure may be attributable to autologous bone flap resorption (when used) or mostly to graft infections,[
Even if CP is a relatively old procedure, there still are no solid evidences on the risk factors for graft infections. The first and most controversial issue is the time interval between DC and CP. Many clinical series state that early CP ensures better outcomes, whereas many others advocate late surgery to prevent graft infections, avoiding the risk of performing surgical procedure on a contaminated wound.[
CONCLUSION
CP is still necessary in surviving patients after life-saving DC. Despite its widespread employment and the wide variety of techniques and material, postoperative complications still threat patients’ clinical outcome. Among these, graft infections are the most common, even many years after the CP have been performed, and they are often due to skin bacterial flora. Nowadays, there is still no consensus on reliable risk factors for early graft infections, moreover late ones are often unpredictable and totally accidental, as we reported. Due to lack of similar cases of very late CP infections in literature, we aimed to share our surgical experience about the most tardive one that we treated, highlighting its extraordinary fortuity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Archavlis E, Carvi Y, Nievas M. The impact of timing in cranioplasty in patients with large cranial defects after decompressive hemicraniectomy. Acta Neurochir (Wien). 2012. 154: 1055-62
2. Benzel EC, Thammavaram K, Kesterson L. The diagnosis of infections associated with acrylic cranioplasties. Neuroradiology. 1990. 32: 151-3
3. Brommeland T, Rydning PN, Pripp AH, Helseth E. Cranioplasty complications and risk factors associated with bone flap resorption. Scand J Trauma Resusc Emerg Med. 2015. 23: 75-
4. Broughton E, pobereskin L, whitfield PC. Seven years of cranioplasty in a regional neurosurgical centre. Br J Neurosurg. 2014. 28: 34-9
5. Cheng YK, Weng HH, Yang JT, Lee MH, Wang TC, Chang CN. Factors affecting graft infection after cranioplasty. J Clin Neurosci. 2008. 15: 1115-9
6. Coulter IC, Pesic-Smith JD, Cato-Addison WB, Khan SA, Thompson D, Jenkins AJ. A multi-centre analysis of the outcomes of cranioplasty in the Northeast of England. Acta Neurochir (Wien). 2014. 156: 1361-8
7. Gooch MR, Gin GE, Kenning TJ, German JW. Complication of cranioplasty following decompressive craniectomy: Analysis of 62 cases. Neurosurg Focus. 2009. 26: E9-
8. Gürbüz MS, Celik O, Berkman MZ. Infection of cranioplasty seen twenty years later. J Korean Neurosurg Soc. 2012. 52: 498-500
9. Graziano F, Certo F, Basile L, Maugeri R, Grasso G, Meccio F. Autologous fibrin sealant (Vivostat(®)) in the neurosurgical practice: Part I: Intracranial surgical procedure. Surg Neurol Int. 2015. 6: 77-
10. Graziano F, Maugeri R, Basile L, Meccio F, Iacopino DG. Aulogous fibrin sealant (Vivostat(®)) in the neurosurgical practice: Part II: Vertebro-spinal procedures. Surg Neurol Int. 2016. 7: S77-82
11. Honeybul S, Ho KM. Cranioplasty: Morbidity and failure. Br J Neurosurg. 2016. 30: 523-8
12. Honeybul S, Janzen C, Kruger K, Ho KM. The impact of cranioplasty on neurological function. Br J Neurosurg. 2013. 27: 636-41
13. Jelcic N, De Pellegrin S, Cecchin D, Della Puppa A, Cagnin A. Cognitive improvement after cranioplasty: A possible volume transmission-related effect. Acta Neurochir (Wien). 2013. 155: 1597-9
14. Kim JS, Park IS, Kim SK, Park H, Kang DH, Lee CH. Analysis of the Risk Factors Affecting the Surgical Site Infection after Cranioplasty Following Decompressive Craniectomy. Korean J Neurotrauma. 2015. 11: 100-15
15. Kimchi G, Stlylianou P, Wohl A, Hadani M, Cohen ZR, Zauberman J. Predicting and reducing cranioplasty infections by clinical, radiographic and operative parameters-A historical cohort study. J Clin Neurosci. 2016. 34: 182-6
16. Klinger DR, Madden C, Beshay J, White J, Gambrell K, Rickert K. Autologous and acrylic cranioplasty: A review of 10 years and 258 cases. World Neurosurg. 2014. 82: 525-30
17. Martin KD, Franz B, Kirsch M, Polanski W, von der Hagen M, Schackert G. Autologous bone flap cranioplasty following decompressive craniectomy is combined with a high complication rate in pediatric traumatic brain injury patients. Acta Neurochir (Wien). 2014. 156: 813-24
18. Maugeri R, Basile L, Giugno A, Graziano F, Iacopino DG. Impasse in the management of recurrent basal cell carcinoma of the skull with sagittal sinus erosion, Interdiscip. Neurosurg. 2015. 2: 160-3
19. Maugeri R, Giammalva GR, Graziano F, Iacopino DG. May autologue fibrin glue alone enhance ossification? An unexpected spinal fusion. World Neurosurg. 2016. 95: 611-2
20. Matsuno A, Tanaka H, Iwamuro H, Takanashi S, Miyawaki S, Nakashima M. Analyses of the factors influencing bone graft infection after delayed cranioplasty. Acta Neurochir (Wien). 2006. 148: 535-40
21. Mattogno PP, LA Rocca G, Signorelli F, Visocchi M. Intracranial subdural empyema: Diagnosis and treatment update. J Neurosurg Sci. 2017. p.
22. Morioka T, Fujiwara S, Akimoto T, Nishio S, Fukui M. Intracranial epidural abscess: Late complication of allograft cranioplasty. Fukuoka Igaku Zasshi. 1996. 87: 57-9
23. Piedra MP, Nemecek AN, Ragel BT. Timing of cranioplasty after decompressive craniectomy for trauma. Surg Neurol Int. 2014. 5: 25-
24. Rosseto RS, Giannetti AV, de Souza Filho LD, Faleiro RM. Risk factors for infection after cranioplasty in patient with large hemicranial bony defects. World Neurosurg. 2015. 84: 431-7
25. Riordan MA, Simpson VM, Hall WA. Analysis of Factors Contributing to Infections After Cranioplasty: A Single-Institution Retrospective Chart Review. World Neurosurg. 2016. 87: 207-13
26. Sari R, Tonge M, Bolukbasi FH, Onoz M, Baskan O, Silav G. Management of failed cranioplasty. Turk Neurosurg. 2017. 27: 201-7
27. Tokoro K, Chiba Y, Tsubone K. Late infection after cranioplasty-review of 14 cases. Neurol Med Chir (Tokyo). 1989. 29: 196-201
28. Visocchi M, Esposito G, Della Pepa GM, Doglietto F, Nucci CG, Fontanella MM. Giant frontal mucocele complicated by subdural empyema: Treatment of a rare association. Acta Neurol Belg. 2012. 112: 85-90
29. Visocchi M, Esposito G, Della Pepa GM, Doglietto F, Nucci CG, Maria Fontanella M. Internal decompressivecraniectomy with craniotomy: A novel surgical therapy of giant frontal mucocele complicated by subdural empyema. Acta Neurol Belg. 2011. 111: 365-70
30. Visocchi M, Mattogno PP, Signorelli F, Zhong J, Iacopino G, Barbagallo G. Complications in Craniovertebral Junction Instrumentation: Hardware Removal Can Be Associated with Long-Lasting Stability. Personal Experience. Acta Neurochir Suppl. 2017. 124: 187-94
31. Yadla S, Campbell PG, Chitale R, Maltenfort MG, Jabbour P, Sharan AD. Effect of early surgery, material, and method of flap preservation on cranioplasty infections: A systematic review. Neurosurgery. 2011. 68: 1124-30
32. Wachter D, Reineke K, Behm T, Rohde V. Cranioplasty after decompressive hemicraniectomy: Underestimated surgery-associated complications?. Clin Neurol Neurosurg. 2013. 115: 1293-7
33. Wiggins A, Austerberry R, Morrison D, Ho KM, Honeybul S. Cranioplasty with custom-made titanium plates-14 years experience. Neurosurgery. 2013. 72: 248-56
34. Wui SH, Kim KM, Ryu YJ, Kim I, Lee SJ, Kim J. The autoclaving of autologous bone is a risk factor for surgical site infection after cranioplasty. World Neurosurg. 2016. 91: 43-9