- Blantyre Malaria Project, University of Malawi College of Medicine, Chichiri, Blantyre, Malawi
- Strong Epilepsy Center, University of Rochester, Rochester, New York, USA
- Department of Osteopathic Medical Specialties, Michigan State University, Michigan, USA
- St. Paul's Eye Unit, Royal Liverpool University Hospital, Liverpool, UK
- School of Medicine, University of St. Andrews, North Haugh, St. Andrews, UK
- Department of Radiology, Michigan State University, Michigan, USA
- Department of Imaging Sciences, Division of Diagnostic and Interventional Neuroradiology, University of Rochester Medical Center, Rochester, New York
Correspondence Address:
Michael J. Potchen
Blantyre Malaria Project, University of Malawi College of Medicine, Chichiri, Blantyre, Malawi
Department of Imaging Sciences, Division of Diagnostic and Interventional Neuroradiology, University of Rochester Medical Center, Rochester, New York
DOI:10.4103/sni.sni_297_17
Copyright: © 2018 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Samuel D. Kampondeni, Gretchen L. Birbeck, Karl B. Seydel, Nicholas A. Beare, Simon J. Glover, Colleen A. Hammond, Cowles A. Chilingulo, Terrie E. Taylor, Michael J. Potchen. Noninvasive measures of brain edema predict outcome in pediatric cerebral malaria. 01-Mar-2018;9:53
How to cite this URL: Samuel D. Kampondeni, Gretchen L. Birbeck, Karl B. Seydel, Nicholas A. Beare, Simon J. Glover, Colleen A. Hammond, Cowles A. Chilingulo, Terrie E. Taylor, Michael J. Potchen. Noninvasive measures of brain edema predict outcome in pediatric cerebral malaria. 01-Mar-2018;9:53. Available from: http://surgicalneurologyint.com/surgicalint-articles/noninvasive-measures-of-brain-edema-predict-outcome-in-pediatric-cerebral-malaria/
Abstract
Background:Increased brain volume (BV) and subsequent herniation are strongly associated with death in pediatric cerebral malaria (PCM), a leading killer of children in developing countries. Accurate noninvasive measures of BV are needed for optimal clinical trial design. Our objectives were to examine the performance of six different magnetic resonance imaging (MRI) BV quantification measures for predicting mortality in PCM and to review the advantages and disadvantages of each method.
Methods:Receiver operator characteristics were generated from BV measures of MRIs of children admitted to an ongoing research project with PCM between 2009 and 2014. Fatal cases were matched to the next available survivor. A total of 78 MRIs of children aged 5 months to 13 years (mean 4.0 years), of which 45% were males, were included.
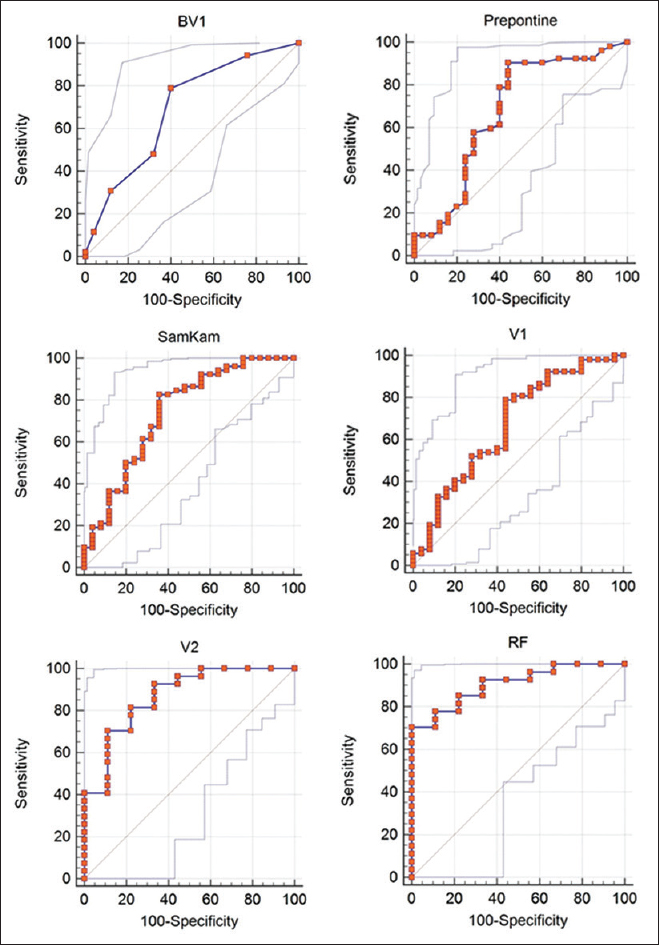
Results:Areas under the curve (AUC) with 95% confidence interval on measures from the initial MRIs were: Radiologist-derived score = 0.69 (0.58–0.79; P = 0.0037); prepontine cistern anteroposterior (AP) dimension = 0.70 (0.56–0.78; P = 0.0133); SamKam ratio [Rt. parietal lobe height/(prepontine AP dimension + fourth ventricle AP dimension)] = 0.74 (0.63–0.83; P = 0.0002); and global cerebrospinal fluid (CSF) space ascertained by ClearCanvas = 0.67 (0.55–0.77; P = 0.0137). For patients with serial MRIs (n = 37), the day 2 global CSF space AUC was 0.87 (0.71–0.96; P P
Conclusion:All noninvasive measures of BV performed well in predicting death and providing a proxy measure for brain volume. Initial MRI assessment may inform future clinical trials for subject selection, risk adjustment, or stratification. Measures of temporal change may be used to stage PCM.
Keywords: Brain herniation, brain volume, intracranial pressure, noninvasive measures, pediatric cerebral malaria, receiver operator characteristic
BACKGROUND
Pediatric Cerebral malaria (PCM) is a leading killer of children in developing countries.[
Objectives
The objectives are to examine the features of the performance of six different MRI BV quantification methods for predicting mortality in PCM using the receiver operator characteristic (ROC) curves and to review the advantages and disadvantages of each method.
MATERIALS AND METHODS
Study design
Retrospective case–control study of children with PCM who had MRIs completed between 2009 and 2014 was conducted comparing survivors and fatal cases.
Inclusion criteria
Exclusion criteria
All cases needed sagittal T1 flair and axial T2 pulse sequences for analysis. Cases without complete sag T1 flair and axial T2 sequences were excluded from the study.
Clinical procedures and image processing
All children underwent fundoscopy by an ophthalmologist to determine retinopathy status. Malarial retinopathy increases the specificity of the clinical diagnosis of cerebral malaria (CM) as it correlates with the presence of cerebral vascular sequestration, the pathological hallmark of CM.[
MRI scanning protocols
Scanning protocols used since 2009 have been published before[
Statistical analysis
The ROC curves for predicting outcome (death vs survival) were derived using six methods-a radiologist derived brain volume, the SamKam ratio,[
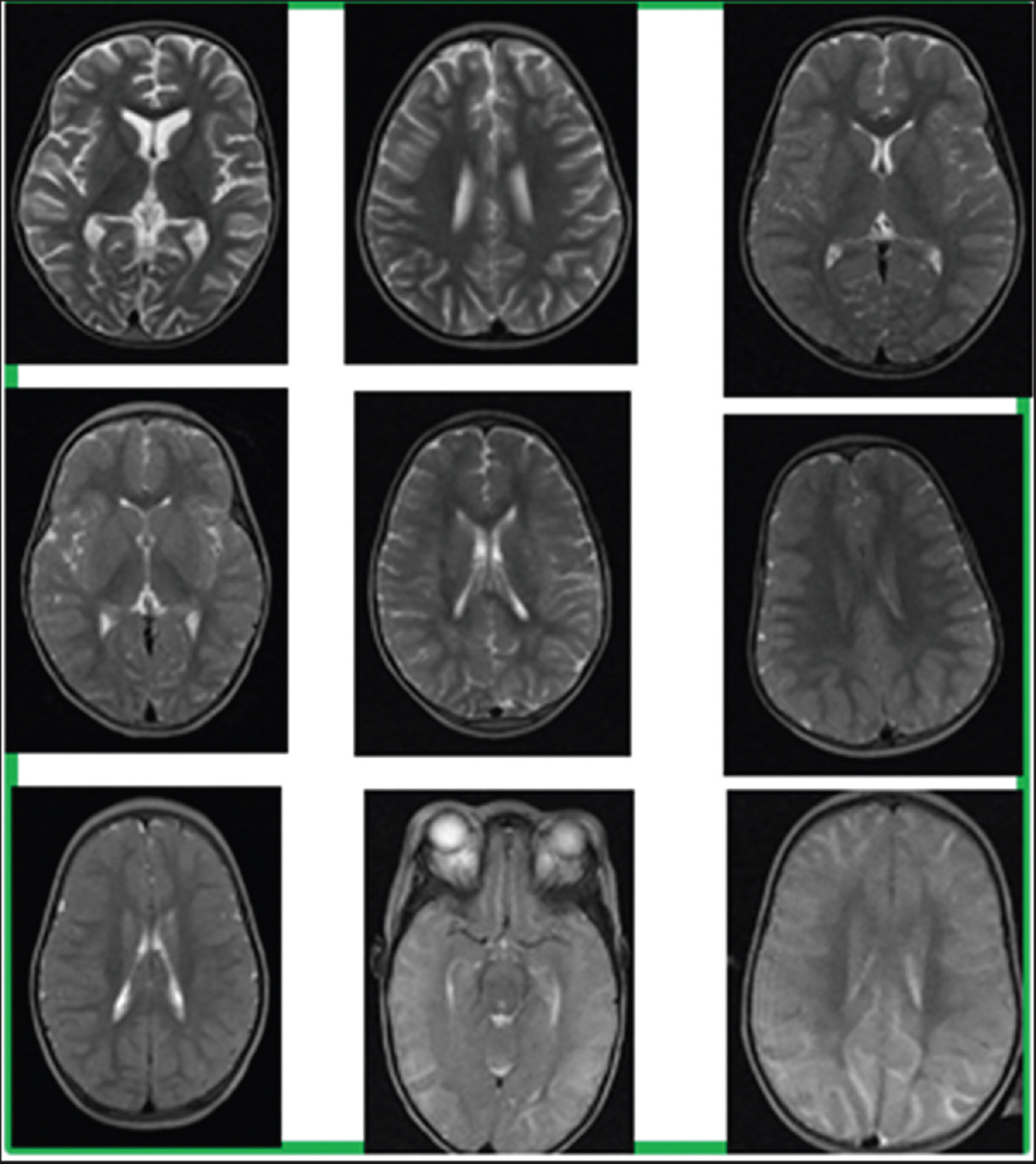
Radiologist-derived brain volume (BV) 1–8 score[ 14 15 ]
This method is performed by an experienced radiologist evaluating the axial T2 pulse sequence images through the cerebral hemispheres [
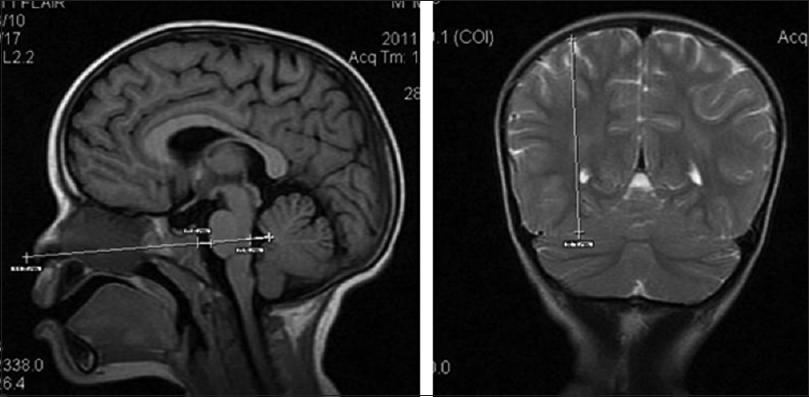
SamKam ratio[ 5 ]
The SamKam ratio is the ratio of the mid-height of the right parietal lobe measured on the first coronal slice behind the splenium of the corpus callosum and the sum of the prepontine cistern and the fourth ventricle anterior–posterior dimensions in the mid-sagittal slice. The rationale of this method is that brain edema expels cranial CSF;[
Prepontine cistern dimension
A linear prepontine cistern anteroposterior dimension in the mid-sagittal plane was used to assess brain edema [
Global CSF Volume Day 1
The cisternal CSF volume around the brainstem was manually quantified by ClearCanvas. Reproducibility of this volume quantification approach has been shown elsewhere.[
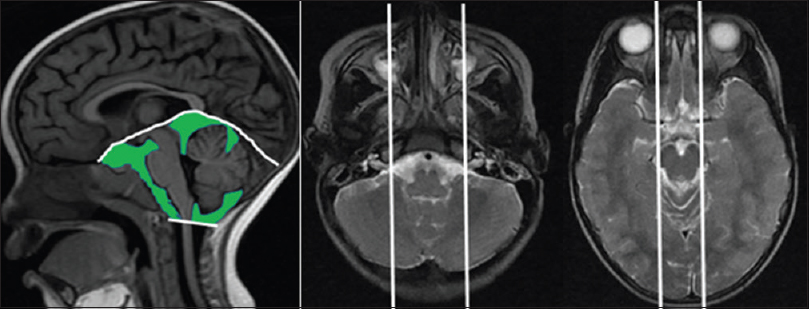
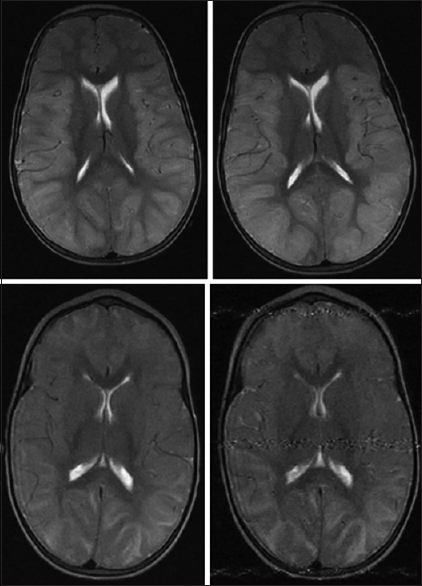
In this method, the cranial cisternal CSF is quantified [
On ClearCanvas, using the sagittal T1 and axial T2 pulse sequences, CSF is quantified on T1 by tracing out the cisternal space areas and the presence of fluid in them is ascertained by cross-referencing with axial T2 slices. The boundaries for sampling are as follows (white lines):
Inferior boundary: Foramen magnum line (left panel) Superior margin: Tentorium cerebelli (left panel) Lateral margins for sampling the cerebellopontine angle cisterns: The sagittal slices through the exits of the 7/8 cranial nerves from the cerebellopontine angle cisterns (middle panel). Lateral margins for sampling the suprasellar and ambient cisterns: The sagittal slices through the lateral margins of the cerebral peduncles (right panel). Pitfalls:
Slow flowing blood causes abnormal T1 signal. It should be included in quantification. The uncus (encroaches into the suprasellar cistern in severe edema); the pituitary stalk and the basilar artery should not be included in quantification. Infratentorial cisternal CSF volume (cubic centimeters) = Sum of cisternal areas × slice thickness.
A subset of children (n = 37) who survived long enough to have a scan on day 2 had brain edema assessed through two approaches–CSF volumes on day 2 and a Recovery Factor (RF) calculation comparing the change in CSF volumes between day 1 and day 2.
Global CSF Volume Day 2
On the second day, the cisternal CSF volume around the brainstem was manually quantified as detailed above for CSF volume day 1.
Recovery factor
The “recovery factor” (RF) variable is the ratio of the global CSF assessments on day 2 and day 1 (RF = Vol. day 2/Vol. day 1). RF indirectly shows the rate of BV change; this is especially significant around the brainstem as compression of vital structures here is directly related to death, both in PCM[
RESULTS
Study population
The study population was n = 78, age range 5 months to 13 years (mean 4 years) with 44.8% males.
ROC curves for predicting outcome (death vs. survival)
The areas under the ROC curves (AUCs) for the six methods were generated using Medcalc software (
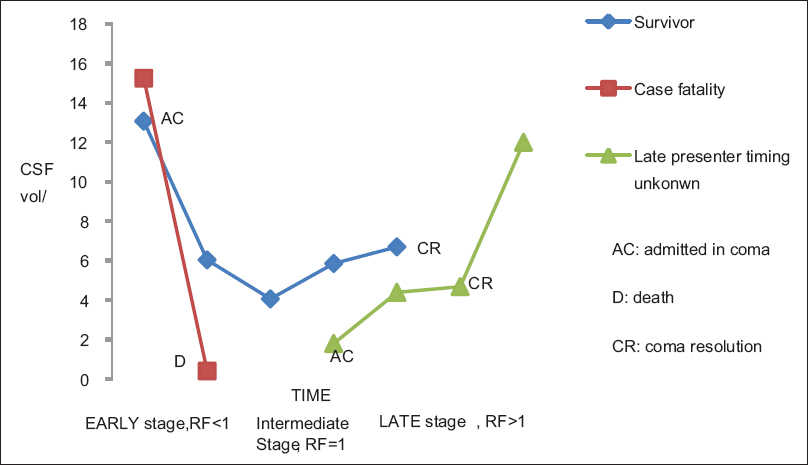
Cranial cisternal CSF volume–time curve; Staging of PCM [ Figure 5 ]
The typical volume–time curve of CSF around the brainstem in survivors of PCM reveals a U-shaped curve. The changing RF values allow staging of PCM into early, intermediate, and late stages. Case fatalities exhibit a sharp decline of CSF volume and their graphs often lack the turn and upswing sections of the curve. Some survivors scanned later in the disease for a variety of reasons may only exhibit the upswing part of the curve.
Grading of PCM severity using RF
The severity of PCM can be graded using the RF. The grading is shown in
Inter-rater agreement on use of the CSF quantification method
A radiologist and an MRI technician assessed a subset of 10 cases to determine the inter-rater agreement for the CSF quantification method. The Pearson coefficient for the two observers was 0.98, suggesting that the method can be reliably used by various observers [Appendices
DISCUSSION
PCM remains a leading killer of African children under the age of five.[
The development of effective brain edema therapies involves monitoring the intracranial space which may be achieved by different methods. In developing countries where malaria is endemic, invasive catheter monitoring cannot be sustained due to high cost, need for highly skilled personnel, and its various complications.[
MRI methods described here accurately predict outcome in PCM. The mortality rates for the various methods are: radiologist BV score of ≥7 (94%); prepontine cistern dimension ≤3 mm (92%); cisternal CSF volume ≥7.5 ml (78 and 80% on day 1 and 2, respectively); and SamKam ratio ≥6.5 (92%). Patients with RF >1 are in the recovery phase, with only 5% mortality from nonedema causes. The mortality rate of those in the early phase is 72% for a RF of ≤0.75 [
Initial MRI assessment may inform clinical trials on patient selection, stratification, and risk adjustment. Serial imaging can stage and grade PCM. Staging PCM has particular relevance in clinical trials because an effective edema therapy needs to alter the slope of the early phase or prolong the intermediate phase. In resource-poor settings, staging may help identify intensive care patients that are recovering from coma earlier than can be determined by global image assessment by radiologists [
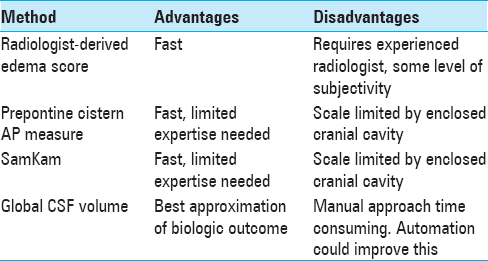
The advantages and disadvantages of the methods described here deserve mention [
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Beare NA, Lewallen S, Taylor TE, Molyneux ME. Redefining cerebral malaria by including malaria retinopathy. Future Microbiol. 2011. 6: 349-55
2. Brinker T, Stopa E, Morrison J, Klinge P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS. 2014. 11: 10-
3. Brott T, Marler JR, Olinger CP, Adams HP, Tomsick T, Barsan WG. Measurements of acute cerebral infarction: Lesion size by computed tomography. Stroke. 1989. 20: 871-5
4. Chesnut RM, Temkin N, Carney N, Dikmen S, Rondina C, Videtta W. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012. 367: 2471-81
5. Kampondeni SD, Chilingulo C, Seydel KB, Potchen MJ, Bradley WG, Latourette M. MRI Measure (SamKam) Predicts Outcome in Pediatric Cerebral Malaria (Abstract No: 1427). Atlanta, GA: American Society of Tropical Medicine and Hygiene; 2012. 87: 433-
6. Khan MN, Shallwani H, Khan MU, Shamim MS. Noninvasive monitoring intracranial pressure-A review of available modalities. Surg Neurol Int. 2017. 8: 51-
7. Lewallen S, Bronzan RN, Beare NA, Harding SP, Molyneux ME, Taylor TE. Using malarial retinopathy to improve the classification of children with cerebral malaria. Trans R Soc Trop Med Hyg. 2008. 102: 1089-94
8. Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V. Indicators of life-threatening malaria in African children. N Engl J Med. 1995. 332: 1399-404
9. Milner DA, Whitten RO, Kamiza S, Carr R, Liomba G, Dzamalala C. The systemic pathology of cerebral malaria in African children. Front Cell Infect Microbiol. 2014. 4: 104-
10. Newton CR, Crawley J, Sowumni A, Waruiru C, Mwangi I, English M. Intracranial hypertension in Africans with cerebral malaria. Arch Dis Child. 1997. 76: 219-26
11. Newton CR, Kirkham FJ, Winstanley PA, Pasvol G, Peshu N, Warrell DA. Intracranial pressure in African children with cerebral malaria. Lancet. 1991. 337: 573-6
12. Newton CR, Peshu N, Kendall B, Kirkham FJ, Sowunmi A, Waruiru C. Brain swelling and ischaemia in Kenyans with cerebral malaria. Arch Dis Child. 1994. 70: 281-7
13. Potchen MJ, Kampondeni SD, Ibrahim K, Bonner J, Seydel KB, Taylor TE. NeuroInterp: A method for facilitating neuroimaging research on cerebral malaria. Neurology. 2013. 81: 585-8
14. Potchen MJ, Kampondeni SD, Seydel KB, Birbeck GL, Hammond CA, Bradley WG. Acute brain MRI findings in 120 Malawian children with cerebral malaria: New insights into an ancient disease. AJNR Am J Neuroradiol. 2012. 33: 1740-6
15. Seydel KB, Kampondeni SD, Valim C, Potchen MJ, Milner DA, Muwalo FW. Brain swelling and death in children with cerebral malaria. N Engl J Med. 2015. 372: 1126-37
16. Smith M. Monitoring intracranial pressure in traumatic brain injury. Anesth Analg. 2008. 106: 240-8
17. Taylor TE. Caring for children with cerebral malaria: Insights gleaned from 20 years on a research ward in Malawi. Trans R Soc Trop Med Hyg. 2009. 103: S6-10
18. Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, Fosiko NG. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004. 10: 143-5
19. Tucker B, Aston J, Dines M, Caraman E, Yacyshyn M, McCarthy M. Early Brain Edema is a Predictor of In-Hospital Mortality in Traumatic Brain Injury. J Emerg Med. 2017. 53: 18-29
20. van der Worp HB, Claus SP, Bar PR, Ramos LM, Algra A, van Gijn J. Reproducibility of measurements of cerebral infarct volume on CT scans. Stroke. 2001. 32: 424-30
21. last accessed on 2018 Feb 1. Available from: http://www.who.int/malaria/publications/world-malaria-report-2017/en/.