- Aga Khan University, Aga Khan University Hospital, Karachi, Pakistan
- Endovascular Research Fellow, University of Buffalo Neurosurgery, Buffalo, New York, USA
- Division of Biological and Biomedical Sciences, Aga Khan University Hospital, Karachi, Pakistan
- Division of Neurosurgery, Aga Khan University Hospital, Karachi, Pakistan
Correspondence Address:
Muhammad Shahzad Shamim
Division of Neurosurgery, Aga Khan University Hospital, Karachi, Pakistan
DOI:10.4103/sni.sni_403_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Marium Naveed Khan, Hussain Shallwani, Muhammad Ulusyar Khan, Muhammad Shahzad Shamim. Noninvasive monitoring intracranial pressure – A review of available modalities. 05-Apr-2017;8:51
How to cite this URL: Marium Naveed Khan, Hussain Shallwani, Muhammad Ulusyar Khan, Muhammad Shahzad Shamim. Noninvasive monitoring intracranial pressure – A review of available modalities. 05-Apr-2017;8:51. Available from: http://surgicalneurologyint.com/surgicalint-articles/noninvasive-monitoring-intracranial-pressure-a-review-of-available-modalities/
Abstract
Background:Intracranial pressure (ICP) monitoring is important in many neurosurgical and neurological patients. The gold standard for monitoring ICP, however, is via an invasive procedure resulting in the placement of an intraventricular catheter, which is associated with many risks. Several noninvasive ICP monitoring techniques have been examined with the hope to replace the invasive techniques. The goal of this paper is to provide an overview of all modalities that have been used for noninvasive ICP monitoring to date.
Methods:A thorough literature search was conducted on PubMed, selected articles were reviewed in completion, and pertinent data was included in the review.
Results:A total of 94 publications were reviewed, and we found that over the past few decades clinicians have attempted to use a number of modalities to monitor ICP noninvasively.
Conclusion:Although the intraventricular catheter remains the gold standard for monitoring ICP, several noninvasive modalities that can be used in settings when invasive monitoring is not possible are also available. In our opinion, measurement of optic nerve sheath diameter and pupillometry are the two modalities which may prove to be valid options for centers not performing invasive ICP monitoring.
Keywords: Intracranial pressure, intracranial pressure monitoring, noninvasive
INTRODUCTION
Intracranial pressure (ICP) is defined as the pressure inside the skull, and therefore, the pressure inside the brain tissue and the cerebrospinal fluid (CSF). The relationship between CSF and intracranial blood volumes is described by the Monroe Kellie doctrine; because the brain is incompressible, when the skull is intact, the sum of the volumes of brain, CSF, and intracranial blood is constant.[
There are several conditions where it is important to monitor ICP, as even minor fluctuations may require a change in management. The gold standard for monitoring ICP is an intraventricular catheter connected to an external pressure transducer; the catheter is placed into one of the ventricles through a burr hole.[
Due to the number of complications associated with invasive ICP monitoring, researchers and clinicians have been trying to develop a reliable noninvasive modality for ICP monitoring. From the use of the Fontogram in the 1970s, to the ongoing experiments on acoustoelasticity effects on ICP, there is still no noninvasive ICP monitoring modality available to replace the invasive techniques.
The aim of this review is to combine a thorough search of all the available noninvasive modalities that have been used to monitor ICP, and to evaluate the feasibility and usefulness of these modalities based on existing literature.
MATERIALS AND METHODS
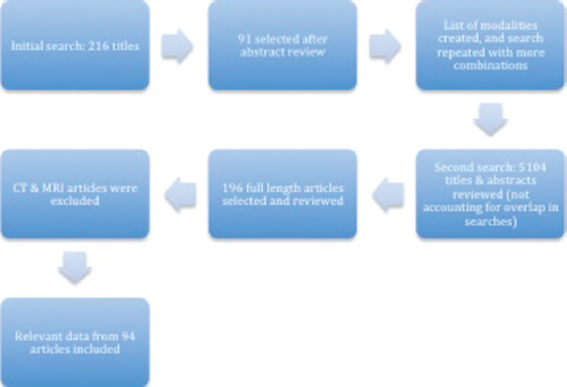
A comprehensive literature search for this review was conducted on PubMed. The search was conducted from November 2014 through to February 2015, and there were no limitations on date, type, or language of the publication. The first search was conducted using the term “non invasive intracranial pressure monitoring,” followed by combination of terms (“intracranial pressure”/”ICP” OR “intracranial pressure monitoring”/”ICP monitoring”) AND (“non-invasive” OR “noninvasive”). These searches provided us with a total of 216 titles. The titles and abstracts were reviewed and 91 publications were selected, based on relevance to our research title, to be reviewed in detail. After reviewing these articles, a list of the noninvasive modalities available to monitor ICP was made, as shown in
The search was then modified to include combinations of “ICP monitoring” or “noninvasive ICP monitoring” AND “anterior fontanelle pressure,” “CT,” “CT scan,” “MRI,” “optic nerve sheath diameter,” “venous ophthalmodynamometry,” “skull elasticity,” “tissue resonance analysis,” “distortion product otoacoustic emissions,” “DPOAE,” “otoacoustic emissions,” “EEG,” “electroencephalography,” “optic disc evaluation,” “ophthalmoscopy,” “papilledema,” “fundoscopy,” “pupillometry,” “neurological pupil index,” and “near infrared spectroscopy.”
The total number of titles and abstracts reviewed after these searches was 5104, not accounting for overlap present in the searches. A total of 196 publications were selected and thoroughly reviewed and read in completion. Our article included relevant data from a total of 94 publications. The length of this paper prevented us from including the role of CT and MRI in monitoring ICP.
Noninvasive intracranial pressure monitoring modalities
Anterior fontanelle pressure monitoring
The anterior fontanelle of the human infant is open, making it an available site to measure ICP in an infant. Many studies were conducted in the 1970s and 1980s to investigate the correlation between pressure application on the anterior fontanelle and the ICP.[
On the same lines, Salmon et al.[
Vidyasagar et al.[
Horbar et al.[
Later, Bunegin et al.[
A more popular method to measure AFP was via the Rotterdam teletransducer (RTT). The RTT is an implantable telemetric device that was introduced by De Jong et al. in 1979 to measure epidural pressure.[
We could neither find any current use of the RTT in monitoring ICP in infants nor any of the other devices for AFP measurements. Moreover, in 2007, Wiegand et al.[
Skull elasticity
Attempts have been made to derive ICP from the mechanical properties of the skull bones. This is based on the hypothesis that, because the skull is not completely rigid, changes in ICP result in a small, but measurable, expansion of the skull.
In 1985, Pitlyk et al.[
In 2009, Yue and Wang[
Optic nerve sheath diameter
The optic nerve sheath, which is continuous with the dura matter of the brain, is surrounded by the subarachnoid space containing CSF.[
On this basis, ocular sonography has been used to measure the changes in ONSD to detect raised ICP, and it has been clinically shown that millimetric increases in the sonographic ONSD corresponds to increased ICP.[
Recent studies have shown that an increase of the ONSD in an estimated range between 4.5 and 5.5 mm is associated with an increased ICP (>20 mmHg).[
Sonographic ONSD measurement is a quick, efficient, and easy to learn modality for the monitoring increased ICP. However, it is important to mention the limitations associated with the measurement of the ONSD as well. Several conditions, including tumors, inflammation, sarcoidosis, and Grave's disease can possibly affect the ONSD, and it is impossible to measure ONSD in those patients with lesions of the orbit or of the optic nerve.[
Venous ophthalmodynamometry
The central retinal vein (CRV) passes through the optic nerve, which as described before is surrounded by CSF, resulting in both the optic nerve and the CRV to be affected by changes in ICP. Therefore, the pressure within the CRV must be as high or higher than the ICP.[
In 1925, Baurmann[
Baurmann[
Querfurth et al.[
In 2011 Firsching et al.[
Like other ophthalmological techniques of monitoring ICP, venous ophthalmodynamometry is a valuable technique to use for screening patients suspected to have increased ICP before carrying out an invasive technique. The method cannot replace invasive techniques, although may be used as a follow-up investigation in some patients.[
Tympanic membrane displacement
Tympanic membrane displacement (TMD) was the first audiologic method studied to monitor ICP noninvasively.[
Samuel et al.[
Stettin et al.[
A recent review article[
Tissue resonance analysis
In 2002, Michaeli et al.[
Based on Michaeli et al’s[
Tonometry
A tonometer is a device used to measure the intraocular pressure (IOP). Several studies have been carried out to test the correlation between IOP and ICP.[
Czarnik et al.[
Most recent studies[
Acoustoelasticity
The acoustoelastic effect describes the effect of a steady stress state on the sound velocities of an elastic material. In 2013, Wu et al.[
Distortion-product otoacoustic emissions
The CSF is continuous with the perilymphatic space, and therefore, changes in ICP can be transmitted to the middle ear.[
In 2006, Voss et al.[
In 2012, Sakka et al.[
Transcranial Doppler
In 1982, Aaslid et al.[
The middle cerebral artery (MCA) is most commonly used for TCD measurements. Both the MCAs are insonated, after which the blood flow is directed towards the probe.[
In a recent review on the role of the TCD,[
In 2004, Bellner et al.[
In 2009, Figaji et al.[
Raguskas et al.[
As mentioned by Kristiansson et al.,[
Electroencephalogram
Initially studies were conducted to assess the role of continuous electroencephalogram (EEG) monitoring for the prognosis of TBI.[
Chen et al.[
Near-infrared spectroscopy
Near-infrared spectroscopy (NIRS) has also been indicated to monitor TBI patients. It can detect changes in cerebral blood volume (CBV), brain tissue oxygenation, and cerebral blood flow (CBF).[
In 1995, Kirkpatrick et al.[
Wagner et al.[
A recent review article[
Pupillometry
In 1983, Marshall et al.[
In 2003, Taylor et al.[
More recently, Chen et al.[
In conclusion, pupillometry is a useful tool for screening patients with possibly increased ICP, however, because conclusive ICP values cannot be detected by this modality, it cannot be suggested for continuous ICP monitoring.
DISCUSSION
Intracranial pressure is elevated in several clinical settings, especially TBI and stroke. The cumulative incidence of these two conditions is approximately 0.6% in developed countries, and their cumulative mortality rate is 30–50%, both of which indicate a major burden of disease.[
We feel that, even though at the moment there is no modality of noninvasive ICP monitoring that can replace invasive ICP monitoring, noninvasive monitoring may still be useful in centers where invasive modalities are not available, such as in developing countries, or even in developed countries as a screening tool to decide, which patients will require invasive monitoring. Invasive ICP monitoring is expensive and requires the availability of a neurosurgeon, both of which are very difficult to acquire in an under resourced regions and also at many trauma centers.[
Of all the different modalities for noninvasive monitoring that we have studied, the authors are of the opinion that two stand out. These are measuring the ONSD and pupillometry. Both modalities are reliable, efficient, affordable, and most importantly, easy to learn. Several studies are ongoing to evaluate their utility in greater depth. Pupillometry, we feel will gain widespread popularity, especially after the introduction of a commercially available, easy to use, nonoperator dependent, electronic pupillometer. Radiology, specifically repeat CT scans for monitoring elevated ICP, has always been, and continues to be an important tool for clinicians, although it was beyond the scope of this paper.
CONCLUSION
Invasive ICP monitoring via a ventricular catheter remains the gold standard, however, there are many areas around the world and several situations where this modality, or other means of invasive ICP monitoring cannot be utilized. Noninvasive modalities provide a useful alternative under such circumstances. Several modalities are available, and even though the ideal modality is yet to be introduced, a number of techniques can be employed. Of these, ONSD and pupillometry may be two modalities to look out for in the future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982. 57: 769-74
2. Amantini A, Carrai R, Lori S, Peris A, Amadori A, Pinto F. Neurophysiological monitoring in adult and pediatric intensive care. Minerva Anestesiol. 2012. 78: 1067-75
3. Amantini A, Fossi S, Grippo A, Innocenti P, Amadori A, Bucciardini L. Continuous EEG-SEP monitoring in severe brain injury. Neurophysiol Clin. 2009. 39: 85-93
4. Ballantyne S, O’Neill G, Hamilton R, Hollman A. Observer variation in the sonographic measurement of optic nerve sheath diameter in normal adults. Eur J Ultrasound. 2002. 15: 145-9
5. Baurmann M. Über die Entstehung und klinische Bedeutung des Netzhautvenenpulses. Dtsch Ophthalmol Ges. 1925. 45: 53-9
6. Behrens A, Lenfeldt N, Ambarki K, Malm J, Eklund A, Koskinen LO. Transcranial Doppler pulsatility index: Not an accurate method to assess intracranial pressure. Neurosurgery. 2010. 66: 1050-7
7. Bellner J, Romner B, Reinstrup P, Kristiansson KA, Ryding E, Brandt L. Transcranial Doppler sonography pulsatility index (PI) reflects intracranial pressure (ICP). Surg Neurol. 2004. 62: 45-51
8. Bershad EM, Urfy MZ, Pechacek A, McGrath M, Calvillo E, Horton NJ. Intracranial pressure modulates distortion product otoacoustic emissions: A proof-of-principle study. Neurosurgery. 2014. 75: 445-54
9. Bohmer A. Hydrostatic pressure in the inner ear fluid compartments and its effects on inner ear function. Acta Otolaryngol Suppl. 1993. 507: 3-24
10. Bouzat P, Francony G, Declety P, Genty C, Kaddour A, Bessou P. Transcranial Doppler to screen on admission patients with mild to moderate traumatic brain injury. Neurosurgery. 2011. 68: 1603-10
11. Bouzat P, Oddo M, Payen JF. Transcranial Doppler after traumatic brain injury: Is there a role?. Curr Opiion in Crit Care. 2014. 20: 153-60
12. Buki B, Avan P, Lemaire JJ, Dordain M, Chazal J, Ribari O. Otoacoustic emissions: A new tool for monitoring intracranial pressure changes through stapes displacements. Hear Res. 1996. 94: 125-39
13. Buki B, de Kleine E, Wit HP, Avan P. Detection of intracochlear and intracranial pressure changes with otoacoustic emissions: A gerbil model. Hear Res. 2002. 167: 180-91
14. Bunegin L, Albin MS, Rauschhuber R, Marlin AE. Intracranial pressure measurement from the anterior fontanelle utilizing a pneumoelectronic switch. Neurosurgery. 1987. 20: 726-731
15. Chen H, Wang J, Mao S, Dong W, Yang H. A new method of intracranial pressure monitoring by EEG power spectrum analysis. Can J Neurol Sci. 2012. 39: 483-7
16. Chen JW, Gombart ZJ, Rogers S, Gardiner SK, Cecil S, Bullock RM. Pupillary reactivity as an early indicator of increased intracranial pressure: The introduction of the Neurological Pupil index. Surg Neurol Int. 2011. 2: 82-
17. Chen JW, Vakil-Gilani K, Williamson KL, Cecil S. Infrared pupillometry, the Neurological Pupil index and unilateral pupillary dilation after traumatic brain injury: Implications for treatment paradigms. SpringerPlus. 2014. 3: 548-
18. Czarnik T, Gawda R, Latka D, Kolodziej W, Sznajd-Weron K, Weron R. Noninvasive measurement of intracranial pressure: Is it possible?. J Trauma. 2007. 62: 207-11
19. de Jong DA, Berfelo MW, de Lange SA, Maas AI. Epidural pressure monitoring with the so-called Rotterdam transducer. Further in vivo results. Acta Neurochir. 1979. 45: 301-9
20. De Jong DA, Maas AI, v d Voort E. Non-invasive intracranial pressure monitoring. A technique for reproducible fontanelle pressure measurements. Z Kinderchir. 1984. 39: 274-6
21. de Riva N, Budohoski KP, Smielewski P, Kasprowicz M, Zweifel C, Steiner LA. Transcranial Doppler pulsatility index: What it is and what it isn’t. Neurocritical Care. 2012. 17: 58-66
22. Deppe C, Kummer P, Gurkov R, Olzowy B. Influence of the individual DPOAE growth behavior on DPOAE level variations caused by conductive hearing loss and elevated intracranial pressure. Ear Hear. 2013. 34: 122-31
23. Djorfe Popovic MK, Stefan Lee. Noninvasive Monitoring of Intracranial Pressure. Recent Patents on Biomedical Engineering. 2009. 2: 165-79
24. Eide PK, Bakken A. The baseline pressure of intracranial pressure (ICP) sensors can be altered by electrostatic discharges. Biomed Eng Online. 2011. 10: 75-
25. Figaji AA, Zwane E, Fieggen AG, Siesjo P, Peter JC. Transcranial Doppler pulsatility index is not a reliable indicator of intracranial pressure in children with severe traumatic brain injury. Surg Neurol. 2009. 72: 389-94
26. Firsching R, Muller C, Pauli SU, Voellger B, Rohl FW, Behrens-Baumann W. Noninvasive assessment of intracranial pressure with venous ophthalmodynamometry. Clinical article. J Neurosurg. 2011. 115: 371-4
27. Firsching R, Schutze M, Motschmann M, Behrens-Baumann W. Venous opthalmodynamometry: A noninvasive method for assessment of intracranial pressure. J Neurosurg. 2000. 93: 33-6
28. Fountas KN, Kapsalaki EZ, Machinis TG, Boev AN, Robinson JS, Troup EC. Clinical implications of quantitative infrared pupillometry in neurosurgical patients. Neurocrit Care. 2006. 5: 55-60
29. Frank AM, Alexiou C, Hulin P, Janssen T, Arnold W, Trappe AE. Non-invasive measurement of intracranial pressure changes by otoacoustic emissions (OAEs)--a report of preliminary data. Zentralbl Neurochir. 2000. 61: 177-80
30. Gaihede M, Felding JU, Elbrônd O. Biomechanical Characteristics of the Middle Ear System Measured by a New Method: III: Comparisons with Tympanometric Measurements. Acta Otolaryngol. 1995. 115: 522-7
31. Geeraerts T, Duranteau J, Benhamou D. Ocular sonography in patients with raised intracranial pressure: The papilloedema revisited. Crit Care. 2008. 12: 150-
32. Geeraerts T, Launey Y, Martin L, Pottecher J, Vigué B, Duranteau J. Ultrasonography of the optic nerve sheath may be useful for detecting raised intracranial pressure after severe brain injury. Intensive Care Med. 2007. 33: 1704-11
33. Ghosh A, Elwell C, Smith M. Review article: Cerebral near-infrared spectroscopy in adults: A work in progress. Anesth Analg. 2012. 115: 1373-1383
34. Gilland O. Normal cerebrospinal-fluid pressure. N Eng J Med. 1969. 280: 904-5
35. Gilland O, Tourtellotte WW, O’Tauma L, Henderson WG. Normal cerebrospinal fluid pressure. J Neurosurg. 1974. 40: 587-93
36. Golan S, Kurtz S, Mezad-Koursh D, Waisbourd M, Kesler A, Halpern P. Poor correlation between intracranial pressure and intraocular pressure by hand-held tonometry. Clin Ophthalmol. 2013. 7: 1083-
37. Gopen Q, Rosowski JJ, Merchant SN. Anatomy of the normal human cochlear aqueduct with functional implications. Hear Res. 1997. 107: 9-22
38. Gura M, Silav G, Isik N, Elmaci I. Noninvasive estimation of cerebral perfusion pressure with transcranial Doppler ultrasonography in traumatic brain injury. Turkish Neurosurg. 2012. 22: 411-5
39. Hayreh SS. Pathogenesis of oedema of the optic disc (pappiloedema).A preliminary report. Br J Ophthalmol. 1964. 48: 522-43
40. Homburg AM, Jakobsen M, Enevoldsen E. Transcranial Doppler recordings in raised intracranial pressure. Acta Neurol Scand. 1993. 87: 488-93
41. Horbar JD, Yeager S, Philip AG, Lucey JF. Effect of application force on noninvasive measurements of intracranial pressure. Pediatrics. 1980. 66: 455-7
42. Killer HE, Laeng HR, Flammer J, Groscurth P. Architecture of arachnoid trabeculae, pillars, and septa in the subarachnoid space of the human optic nerve: Anatomy and clinical considerations. Br J Ophthalmol. 2003. 87: 777-81
43. Kim YK, Seo H, Yu J, Hwang GS. Noninvasive estimation of raised intracranial pressure using ocular ultrasonography in liver transplant recipients with acute liver failure -A report of two cases. Korean J Anesthesiol. 2013. 64: 451-5
44. Kimberly HH, Shah S, Marill K, Noble V. Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med. 2008. 15: 201-4
45. Kirkpatrick PJ, Smielewski P, Czosnyka M, Menon DK, Pickard JD. Near-infrared spectroscopy use in patients with head injury. J Neurosurg. 1995. 83: 963-70
46. Klingelhöfer J, Conrad B, Benecke R, Sander D. Intracranial flow patterns at increasing intracranial pressure. Klin Wochenschr. 1987. 65: 542-5
47. Klingelhöfer J, Conrad B, Benecke R, Sander D, Markakis E. Evaluation of intracranial pressure from transcranial Doppler studies in cerebral disease. J Neurol. 1988. 235: 159-62
48. Krishnamoorthy V, Beckmann K, Mueller M, Sharma D, Vavilala MS. Perioperative estimation of the intracranial pressure using the optic nerve sheath diameter during liver transplantation. Liver Transpl. 2013. 19: 246-9
49. Kristiansson H, Nissborg E, Bartek J, Andresen M, Reinstrup P, Romner B. Measuring elevated intracranial pressure through noninvasive methods: A review of the literature. J Neurosurg Anesthesiol. 2013. 25: 372-85
50. Lashutka MK, Chandra A, Murray HN, Phillips GS, Hiestand BC. The relationship of intraocular pressure to intracranial pressure. Ann Emerg Med. 2004. 43: 585-91
51. Lescot T, Naccache L, Bonnet MP, Abdennour L, Coriat P, Puybasset L. The relationship of intracranial pressure Lundberg waves to electroencephalograph fluctuations in patients with severe head trauma. Acta Neurochir. 2005. 147: 125-9
52. Li Z, Yang Y, Lu Y, Liu D, Xu E, Jia J. Intraocular pressure vs intracranial pressure in disease conditions: A prospective cohort study (Beijing iCOP study). BMC Neurol. 2012. 12: 66-
53. Marchbanks RJ. Measurement of tympanic membrane displacement arising from aural cardiovascular activity, swallowing, and intra-aural muscle reflex. Acta Otolaryngol. 1984. 98: 119-29
54. Marshall LF, Barba D, Toole BM, Bowers SA. The oval pupil: Clinical significance and relationship to intracranial hypertension. J Neurosurg. 1983. 58: 566-8
55. Michaeli D, Rappaport ZH. Tissue resonance analysis; a novel method for noninvasive monitoring of intracranial pressure. Technical note. J Neurosurg. 2002. 96: 1132-7
56. Miller MT, Pasquale M, Kurek S, White J, Martin P, Bannon K. Initial head computed tomographic scan characteristics have a linear relationship with initial intracranial pressure after trauma. J Trauma. 2004. 56: 967-72
57. Mokri B. The Monro-Kellie hypothesis: Applications in CSF volume depletion. Neurology. 2001. 56: 1746-8
58. Moreno JA, Mesalles E, Gener J, Tomasa A, Ley A, Roca J. Evaluating the outcome of severe head injury with transcranial Doppler ultrasonography. Neurosurg Focus. 2000. 8: 1-7
59. Murakami S, Gyo K, Goode RL. Effect of increased inner ear pressure on middle ear mechanics. Otolaryngol Head Neck Surg. 1998. 118: 703-8
60. Overweg-Plandsoen WCG. Anterior fontanelle pressure monitoring in infants [Ph.D. thesis]: Erasmus University Rotterdam. 1990. p.
61. Pitlyk PJ, Piantanida TP, Ploeger DW. Noninvasive intracranial pressure monitoring. Neurosurgery. 1985. 17: 581-4
62. Plandsoen WC, de Jong DA, Maas AI, Stroink H, Avezaat CJ. Fontanelle pressure monitoring in infants with the Rotterdam Teletransducer: A reliable technique. Med Prog Technol. 1987. 13: 21-7
63. Querfurth HW, Arms SW, Lichy CM, Irwin WT, Steiner T. Prediction of intracranial pressure from noninvasive transocular venous and arterial hemodynamic measurements: A pilot study. Neurocrit Care. 2004. 1: 183-94
64. Raboel PH, Bartek J, Andresen M, Bellander BM, Romner B. Intracranial Pressure Monitoring: Invasive versus Non-Invasive Methods-A Review. Crit Care Res Pract 2012. 2012. p. 950393-
65. Ragauskas A, Matijosaitis V, Zakelis R, Petrikonis K, Rastenyte D, Piper I. Clinical assessment of noninvasive intracranial pressure absolute value measurement method. Neurology. 2012. 78: 1684-91
66. Rainov N, Weise JB, Burkert W. Transcranial Doppler sonography in adult hydrocephalic patients. Neurosurg Rev. 2000. 23: 34-8
67. Rajajee V, Vanaman M, Fletcher JJ, Jacobs TL. Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocrit Care. 2011. 15: 506-15
68. Raksin PB, Alperin N, Sivaramakrishnan A, Surapaneni S, Lichtor T. Noninvasive intracranial compliance and pressure based on dynamic magnetic resonance imaging of blood flow and cerebrospinal fluid flow: Review of principles, implementation, and other noninvasive approaches. Neurosurg Focus. 2003. 14: e4-
69. Reid A, Marchbanks R, Burge D, Martin A, Bateman D, Pickard J. The relationship between intracranial pressure and tympanic membrane displacement. Br J Audiol. 1990. 24: 123-9
70. Rosenberg JB, Shiloh AL, Savel RH, Eisen LA. Non-invasive methods of estimating intracranial pressure. Neurocrit Care. 2011. 15: 599-608
71. Sajjadi SA, Harirchian MH, Sheikhbahaei N, Mohebbi MR, Malekmadani MH, Saberi H. The relation between intracranial and intraocular pressures: Study of 50 patients. Ann Neurol. 2006. 59: 867-70
72. Sakka L, Thalamy A, Giraudet F, Hassoun T, Avan P, Chazal J. Electrophysiological monitoring of cochlear function as a non-invasive method to assess intracranial pressure variations. Acta Neurochir Suppl. 2012. 114: 131-4
73. Salmon JH, Hajjar W, Bada HS. The fontogram: A noninvasive intracranial pressure monitor. Pediatrics. 1977. 60: 721-5
74. Samuel M, Burge DM, Marchbanks RJ. Tympanic membrane displacement testing in regular assessment of intracranial pressure in eight children with shunted hydrocephalus. J Neurosurg. 1998. 88: 983-95
75. Sheeran P, Bland J, Hall G. Intraocular pressure changes and alterations in intracranial pressure. Lancet. 2000. 355: 899-
76. Smith M. Monitoring intracranial pressure in traumatic brain injury. Anesth Analg. 2008. 106: 240-8
77. Soldatos T, Chatzimichail K, Papathanasiou M, Gouliamos A. Optic nerve sonography: A new window for the non-invasive evaluation of intracranial pressure in brain injury. Emerg Med J. 2009. 26: 630-4
78. Soldatos T, Karakitsos D, Chatzimichail K, Papathanasiou M, Gouliamos A, Karabinis A. Optic nerve sonography in the diagnostic evaluation of adult brain injury. Crit Care. 2008. 12: R67-
79. Stettin E, Paulat K, Schulz C, Kunz U, Mauer UM. Noninvasive intracranial pressure measurement using infrasonic emissions from the tympanic membrane. J Clin Monit Comput. 2011. 25: 203-10
80. Strumwasser A, Kwan RO, Yeung L, Miraflor E, Ereso A, Castro-Moure F. Sonographic optic nerve sheath diameter as an estimate of intracranial pressure in adult trauma. J Surg Res. 2011. 170: 265-71
81. Tayal VS, Neulander M, Norton HJ, Foster T, Saunders T, Blaivas M. Emergency department sonographic measurement of optic nerve sheath diameter to detect findings of increased intracranial pressure in adult head injury patients. Ann Emerg Med. 2007. 49: 508-14
82. Taylor WR, Chen JW, Meltzer H, Gennarelli TA, Kelbch C, Knowlton S. Quantitative pupillometry, a new technology: Normative data and preliminary observations in patients with acute head injury. Technical note. J Neurosurg. 2003. 98: 205-13
83. Chunyu T, XP , Li N, Qin L, Tian Z. The Correlation between Intracranial Pressure and Intraocular Pressure after Brain Surgery. Int J Ophthalmol Eye Res. 2014. 2: 54-8
84. Vidyasagar D, Raju TN. A simple noninvasive technique of measuring intracranial pressure in the newborn. Pediatrics. 1977. 59: 957-61
85. Voss SE, Adegoke MF, Horton NJ, Sheth KN, Rosand J, Shera CA. Posture systematically alters ear-canal reflectance and DPOAE properties. Hear Res. 2010. 263: 43-51
86. Voss SE, Horton NJ, Tabucchi TH, Folowosele FO, Shera CA. Posture-induced changes in distortion-product otoacoustic emissions and the potential for noninvasive monitoring of changes in intracranial pressure. Neurocrit Care. 2006. 4: 251-7
87. Voulgaris SG, Partheni M, Kaliora H, Haftouras N, Pessach IS, Polyzoidis KS. Early cerebral monitoring using the transcranial Doppler pulsatility index in patients with severe brain trauma. Med Sci Monit. 2005. 11: CR49-52
88. Wagner BP, Pfenninger J. Dynamic cerebral autoregulatory response to blood pressure rise measured by near-infrared spectroscopy and intracranial pressure. Crit Care Med. 2002. 30: 2014-21
89. Wakerley B, Yohana K, Luen Teoh H, Tan CW, Chan BP, Sharma VK. Non-invasive intracranial pressure monitoring with transcranial Doppler in a patient with progressive cerebral venous sinus thrombosis. J Neuroimaging. 2014. 24: 302-4
90. Wakerley BR, Kusuma Y, Yeo LL, Liang S, Kumar K, Sharma AK. Usefulness of Transcranial Doppler-Derived Cerebral Hemodynamic Parameters in the Noninvasive Assessment of Intracranial Pressure. J Neuroimaging. 2015. 25: 111-6
91. Wiegand C, Richards P. Measurement of intracranial pressure in children: A critical review of current methods. Dev Med Child Neurol. 2007. 49: 935-41
92. Wu J, He W, Chen WM, Zhu L. Research on simulation and experiment of noninvasive intracranial pressure monitoring based on acoustoelasticity effects. Med Devices. 2013. 6: 123-31
93. Xianfang Yue LW. Deformation of skull bone as intracranial pressure changing. African Journal of Biotechnology. 2009. 8: 745-50
94. Zweifel C, Czosnyka M, Carrera E, de Riva N, Pickard JD, Smielewski P. Reliability of the blood flow velocity pulsatility index for assessment of intracranial and cerebral perfusion pressures in head-injured patients. Neurosurgery. 2012. 71: 853-61