- Department of Neurosurgery, Emory University School of Medicine, Atlanta, Georgia
- Faculty of Health, University of Canberra, Canberra, Australia
- Department of Neurosciences, Centura Health Physician Group Neuroscience and Spine, Lakewood, Colorado, USA
Correspondence Address:
Alexander Mason
Department of Neurosurgery, Emory University School of Medicine, Atlanta, Georgia
DOI:10.4103/sni.sni_452_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nefize Turan, Shannon Butler, Theodore C. Larson, Alexander Mason. Nontraumatic, posterior circulation pseudoaneurysm of the basilar artery summit with complete spontaneous resolution: Case report and literature review. 05-Apr-2017;8:50
How to cite this URL: Nefize Turan, Shannon Butler, Theodore C. Larson, Alexander Mason. Nontraumatic, posterior circulation pseudoaneurysm of the basilar artery summit with complete spontaneous resolution: Case report and literature review. 05-Apr-2017;8:50. Available from: http://surgicalneurologyint.com/surgicalint-articles/nontraumatic-posterior-circulation-pseudoaneurysm-of-the-basilar-artery-summit-with-complete-spontaneous-resolution-case-report-and-literature-review/
Abstract
Background:Intracranial pseudoaneurysms are rare vascular defects of arterial walls that are classically the result of traumatic injury, iatrogenic causes, or infection. Idiopathic pseudoaneurysms are seen even less frequently and are often related to atherosclerosis. Pseudoaneurysms are most commonly found along the distal wall of the internal carotid artery, however, can occur at any location in the cerebrovascular circulation. Treatment of these arterial defects is often challenging due to their frail nature.
Case Description:A 61-year-old male with a history of hypertension presented with a severe, atypical headache without history of trauma. Computed tomography (CT) and computed tomography angiography (CTA) demonstrated diffuse subarachnoid hemorrhage. Imaging demonstrated a 3.5 mm pseudoaneurysm projecting distally from the basilar artery at the apex. Repeated imaging (CTA, digital subtraction angiography) demonstrated decreased size and flow associated within the aneurysm over the following 2 weeks; as such, the patient was managed conservatively. The patient was discharged in neurologically intact condition when imaging at 14 days confirmed complete and spontaneous resolution of the pseudoaneurysm.
Conclusion:Idiopathic pseudoaneurysms that are commonly associated with atherosclerosis are most commonly managed surgically or endovascularly. Conservative approach may be considered in a select group of patients that exhibit decreased size and/or flow within the aneurysm in repeated imaging; spontaneous resolution was seen in the present case.
Keywords: Basilar artery, conservative management, pseudoaneurysm, subarachnoid hemorrhage
INTRODUCTION
Intracranial pseudoaneurysms are potentially dangerous arterial lesions classically caused by trauma,[
Unlike true aneurysms, pseudoaneurysms are not typically found on the bifurcation of vessels, but rather along a vessel wall, distal from a branch point.[
Pseudoaneurysms can occur on vessels in posterior circulation, as is our case, however, they most typically occur in anterior circulation on the internal carotid artery (ICA), and are also known as blood blister-like aneurysms. Nomenclature in the literature uses both terms interchangeably. Treatment is often challenging because of the frail nature of these false aneurysms, which have a tendency to rupture during treatment.[
CASE REPORT
History and examination
A 61-year-old male with a history of hypertension presented to the emergency department with a severe, atypical headache without a history of trauma. The patient was neurologically intact, Hunt and Hess grade II, with an initial blood pressure of 172/96 mmHg.
Imaging
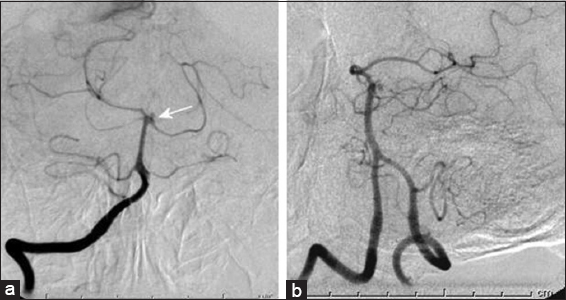
Computed tomography (CT) and subsequent computed tomography angiography (CTA) demonstrated diffuse SAH, contained largely within the basilar cisterns, but without clear source of the hemorrhage on CTA. Subsequent digital subtraction angiography (DSA) and digital rotational angiography (DRA) demonstrated a 3.5 mm idiopathic pseudoaneurysm projecting posteriorly from the basilar summit/posterior cerebral artery junction without evidence of arterial dissection [
Intensive care unit course and management
Subsequent imaging both with CTA and DSA/DRA demonstrated decreased size and flow associated within the aneurysm. Expectant management of the aneurysm was undertaken and no subsequent treatment was administered. The patient had an expected course in the intensive care unit (ICU), including temporary ventricular drainage, and was discharged home in neurologically intact in stable condition. Follow-up DSA and DRA 14 days after hemorrhage demonstrated complete resolution of the pseudoaneurysm [
DISCUSSION
“False aneurysms” have been described in the literature as early as 1928 due to trauma and iatrogenic causes. In a case series by Besser et al., approximately 5% of the vertebrobasilar aneurysms were described as “false aneurysms.”[
Idiopathic (nontraumatic and noninfectious) pseudoaneurysms, one of which is presented in the current case report, are relatively rare compared with pseudoaneurysms that are secondary to trauma,[
Patients with this condition typically present with signs and symptoms consistent with SAH and include severe, thunderclap headache.[
Treatment is typically necessary to repair the site of rupture of the pseudoaneurysm to avoid further subarachnoid hemorrhage and subsequent complications. Because a pseudoaneurysm is, by definition, a complete laceration or avulsion of all three layers of a vessel wall, they may be best treated as lesions whereby rupture or re-rupture is imminent. Typical variables to consider when selecting a treatment method include cause, location, and comfort of the treating physician with various treatment modalities. Because the procedural rupture rate is high regardless of the treatment method, multiple modalities for treatment should be considered.
Three modes of treatment for pseudoaneurysms are open surgical, endovascular techniques, and conservative management. Surgical techniques that have been used to treat pseudoaneurysms include clipping, wrapping, clipping followed by wrapping, wrapping followed by clipping, and suturing. There have been multiple cases in which an encircling (Sugita) clip was successful in the treatment of an ICA and ACoA pseudoaneurysms.[
The patient in the present case report was treated conservatively due to decreased size and flow of the pseudoaneurysm in repeat CTA and DSA/DRA compared with the initial images. He was discharged from the ICU in neurologically intact condition when imaging at fourteen days revealed complete spontaneous resolution of the pseudoaneurysm. Spontaneous resolution of pseudoaneurysms is considered to be a rare phenomenon.[
In the present case, both 14-day and 1-year follow-up imaging showed consistent resolution. Moreover, spontaneous resolution of basilar artery perforator aneurysms has also been reported suggesting that aneurysms in the posterior circulation, possibly due to different flow patterns, may possibly undergo spontaneous resolution more frequently.[
CONCLUSION
The present case is, to the best of our knowledge, the first reported incident of spontaneous resolution of an idiopathic pseudoaneurysm in the posterior circulation. All other reported incidences of spontaneous resolution have occurred where the pseudoaneurysm was caused by recent head trauma. None of these patients, including the present case, exhibited atherosclerosis of the parent vessel—this contrasts with the vast majority of pseudoaneurysm cases reviewed, in which treatment was necessary and atherosclerosis was present in both the parent vessel and the circle of Willis.
Treatment for an intracranial pseudoaneurysm should be carefully considered, and be based on the location of the aneurysm and the comfort level of a surgeon to perform a specific procedure deemed most appropriate. A conservative approach may be considered for selected pseudoaneurysms where potentially atherosclerosis is not present and where consecutive imaging shows a decrease in size and flow of the aneurysm.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abe M, Tabuchi K, Yokoyama H, Uchino A. Blood blisterlike aneurysms of the internal carotid artery. J Neurosurg. 1998. 89: 419-24
2. Baskaya MK, Ahmed AS, Ates O, Niemann D. Surgical treatment of blood blister-like aneurysms of the supraclinoid internal carotid artery with extracranial-intracranial bypass and trapping. Neurosurg Focus. 2008. 24: E13-
3. Benoit BG, Wortzman G. Traumatic cerebral aneurysms. Clinical features and natural history. J Neurol Neurosurg Psychiatry. 1973. 36: 127-38
4. Besser M, McKechnie SA. Surgical management of vertebrobasilar aneurysms. J Clin Neurosci. 1997. 4: 39-46
5. Charbel FT, Gonzales-Portillo G, Hoffman W, Cochran E. Distal internal carotid artery pseudoaneurysms: Technique and pitfalls of surgical management: Two technical case reports. Neurosurgery. 1999. 45: 643-8
6. Chavent A, Lefevre PH, Thouant P, Cao C, Kazemi A, Mourier K. Spontaneous resolution of perforator aneurysms of the posterior circulation. J Neurosurg. 2014. 121: 1107-111
7. Chung JH, Shin YS, Lim YC, Park M. Ideal Internal Carotid Artery Trapping Technique without Bypass in a Patient with Insufficient Collateral Flow. J Korean Neurosurg Soc. 2009. 45: 260-3
8. Ding H, You C, Yin H. Nontraumatic and noninfectious pseudoaneurysms on the circle of Willis: 2 case reports and review of the literature. Surg Neurol. 2008. 69: 414-7
9. Honda M, Nagamine T, Yamashiro K, Shimoji T. An intracranial pseudoaneurysm in the distal middle cerebral artery in a child. Pediatr Neurosurg. 2008. 44: 426-9
10. Jadhav AP, Pryor JC, Nogueira RG. Onyx embolization for the endovascular treatment of infectious and traumatic aneurysms involving the cranial and cerebral vasculature. J Neurointerv Surg. 2013. 5: 562-5
11. Kim T, Bang JS, Hwang G, Kwon OK, Oh CW, Nam KH. Idiopathic lenticulostriate artery pseudoaneurysm protruding into the lateral ventricle: A case report. J Cerebrovasc Endovasc Neurosurg. 2013. 15: 246-50
12. Korja M, Rautio R, Valtonen S, Haapanen A. Primary treatment of ruptured blood blister-like aneurysms with stent-assisted coil embolization: Report of two cases. Acta Radiol. 2008. 49: 180-3
13. Kubo Y, Ogasawara K, Tomitsuka N, Otawara Y, Watanabe M, Ogawa A. Wrap-clipping with polytetrafluoroethylene for ruptured blisterlike aneurysms of the internal carotid artery. Technical note. J Neurosurg. 2006. 105: 785-7
14. Le Feuvre DE, Taylor AG. The management of very small/blister internal carotid artery aneurysms. Interv Neuroradiol. 2011. 17: 431-4
15. Lee BH, Kim BM, Park MS, Park SI, Chung EC, Suh SH. Reconstructive endovascular treatment of ruptured blood blister-like aneurysms of the internal carotid artery. J Neurosurg. 2009. 110: 431-6
16. Lee JW, Choi HG, Jung JY, Huh SK, Lee KC. Surgical strategies for ruptured blister-like aneurysms arising from the internal carotid artery: A clinical analysis of 18 consecutive patients. Acta Neurochir. 2009. 151: 125-30
17. Lister JR, Sypert GW. Traumatic false aneurysm and carotid-cavernous fistula: A complication of sphenoidotomy. Neurosurgery. 1979. 5: 473-5
18. Loevner LA, Ting TY, Hurst RW, Goldberg HI, Schut L. Spontaneous thrombosis of a basilar artery traumatic aneurysm in a child. AJNR Am J Neuroradiol. 1998. 19: 386-8
19. McElroy KM, Malone RJ, Freitag WB, Keller I, Shepard S, Roychowdhury S. Traumatic pseudoaneurysm of the basilar artery. Am J Phys Med Rehabil. 2008. 87: 690-1
20. Molyneux AJ, Kerr RS, Birks J, Ramzi N, Yarnold J, Sneade M. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): Long-term follow-up. Lancet Neurol. 2009. 8: 427-33
21. Morgan M, Besser M, Tuck R. Pseudoaneurysm complicating superficial temporal artery-superior cerebellar artery bypass. Surg Neurol. 1986. 26: 277-81
22. Nakstad P. Spontaneous occlusion of traumatic pericallosal aneurysm and pericallosal artery. Neuroradiology. 1987. 29: 312-
23. Nimjee SM, Smith TP, Kanter RJ, Ames W, Machovec KA, Grant GA. Rapid ventricular pacing for a basilar artery pseudoaneurysm in a pediatric patient: Case report. J Neurosurg Pediatr. 2015. 15: 625-9
24. Ogawa A, Suzuki M, Ogasawara K. Aneurysms at nonbranching sites in the surpaclinoid portion of the internal carotid artery: Internal carotid artery trunk aneurysms. Neurosurgery. 2000. 47: 578-83
25. Otani N, Takasato Y, Masaoka H, Hayakawa T, Yoshino Y, Yatsushige H. Clinical and radiological findings and surgical management of ruptured aneurysms at the non-branching sites of the internal carotid artery. J Clin Neurosci. 2009. 16: 1018-23
26. Park JH, Park IS, Han DH, Kim SH, Oh CW, Kim JE. Endovascular treatment of blood blister-like aneurysms of the internal carotid artery. J Neurosurg. 2007. 106: 812-9
27. Pelz DM, Ferguson GG, Lownie SP, Kachur E. Combined endovascular/neurosurgical therapy of blister-like distal internal carotid aneurysms. Can J Neurol Sci. 2003. 30: 49-53
28. Quintana F, Diez C, Gutierrez A, Diez ML, Austin O, Vazquez A. Traumatic aneurysm of the basilar artery. AJNR Am J Neuroradiol. 1996. 17: 283-5
29. Rowed DW, Walters BC. Iatrogenic false aneurysm following repair of intracranial aneurysm. Can J Neurol Sci. 1994. 21: 346-9
30. Sakata N, Takebayashi S, Kojima M, Masawa N, Suzuki K, Takatama M. Different roles of arteriosclerosis in the rupture of intracranial dissecting aneurysms. Histopathology. 2001. 38: 325-37
31. Shah Q, Friedman J, Mamourian A. Spontaneous resolution of traumatic pseudoaneurysm of the middle meningeal artery. AJNR Am J Neuroradiol. 2005. 26: 2530-2
32. Srinivasan A, Lesiuk H, Goyal M. Spontaneous resolution of posttraumatic middle meningeal artery pseudoaneurysm. AJNR Am J Neuroradiol. 2006. 27: 882-3
33. Takemoto Y, Hasegawa S, Nagamine M, Kasamo D, Matsumoto J, Miura M. A spontaneous superficial temporal artery pseudoaneurysm possibly related to atherosclerosis: Case report and review of literature. Surg Neurol Int. 2016. 7: S247-50
34. Tanoue S, Kiyosue H, Matsumoto S, Yamashita M, Nagatomi H, Mori H. Ruptured “blisterlike” aneurysm with a pseudoaneurysm formation requiring delayed intervention with endovascular coil embolization. Case report. J Neurosurg. 2004. 101: 159-62
35. Teitelbaum GP, Bernstein K, Choi S, Giannotta SL. Endovascular coil occlusion of a traumatic basilar-cavernous fistula: Technical report. Neurosurgery. 1998. 42: 1394-7
36. Tekiner A, Gokcek C, Bayar MA, Erdem Y, Kilic C. Spontaneus resolution of a traumatic vertebral artery pseudoaneurysm. Turk Neurosurg. 2011. 21: 90-3
37. Wang X, Chen JX, You C, He M. Surgical management of traumatic intracranial pseudoaneurysms: A report of 12 cases. Neurol India. 2008. 56: 47-51
38. Zanaty M, Chalouhi N, Jabbour P, Starke RM, Hasan D. The unusual angiographic course of intracranial pseudoaneurysms. Asian J Neurosurg. 2015. 10: 327-30