- Department of Oncology, Montefiore Medical Center, New York, USA
- Department of Neurosuregry, Montefiore Medical Center, New York, USA
- Department of Pathology, Montefiore Medical Center, New York, USA
- Department of Neurology, Montefiore Medical Center, New York, USA
Correspondence Address:
Jonathan Nakhla
Department of Neurology, Montefiore Medical Center, New York, USA
DOI:10.4103/2152-7806.189731
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Gajavelli S, Nakhla J, Nasser R, Yassari R, Weidenheim KM, Graber J. Ollier disease with anaplastic astrocytoma: A review of the literature and a unique case. Surg Neurol Int 01-Sep-2016;7:
How to cite this URL: Gajavelli S, Nakhla J, Nasser R, Yassari R, Weidenheim KM, Graber J. Ollier disease with anaplastic astrocytoma: A review of the literature and a unique case. Surg Neurol Int 01-Sep-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/ollier-disease-anaplastic-astrocytoma-review-literature-unique-case/
Abstract
Background:Ollier disease is a rare, nonfamilial disorder that primary affects the long bones and cartilage of joints with multiple enchondromas. It is associated with a higher risk of central nervous system (CNS) malignancies; although the incidence is unknown.
Case Description:Here, we present the case of a 55-year-old woman who developed an anaplastic astrocytoma with a known diagnosis of Ollier disease with a survival time of over 3 years.
Conclusion:This report draws attention to the rarity of this disease and the paucity of information regarding CNS involvement in Ollier disease, as well as reviews the current literature.
Keywords: Astrocytoma, endochondroma, IDH1 mutation, intracranial tumor, Ollier Disease
INTRODUCTION
Ollier disease is a rare, nonfamilial disorder with a prevalence of 1:100,000, characterized by multiple enchondromatosis, with an asymmetric distribution, and areas of dysplastic cartilage. The condition primarily affects the long bones and cartilage of the joints of the arms and legs, specifically at the metaphysis and is divided into 6 types.[
It remains uncertain whether the disorder is caused by a single gene defect or by combinations of (germline and/or somatic) mutations.[
Clinical manifestations often appear in the first decade of life and usually start with local pain, bone swelling, and palpable bony masses, which is often associated with bone deformity. Headache and cranial nerve palsy are prominent clinical findings. The only effective treatment is the surgical resection of the lesions and symptomatic treatment of the possible complications such as pathological fractures, growth defect, and neurological symptoms.[
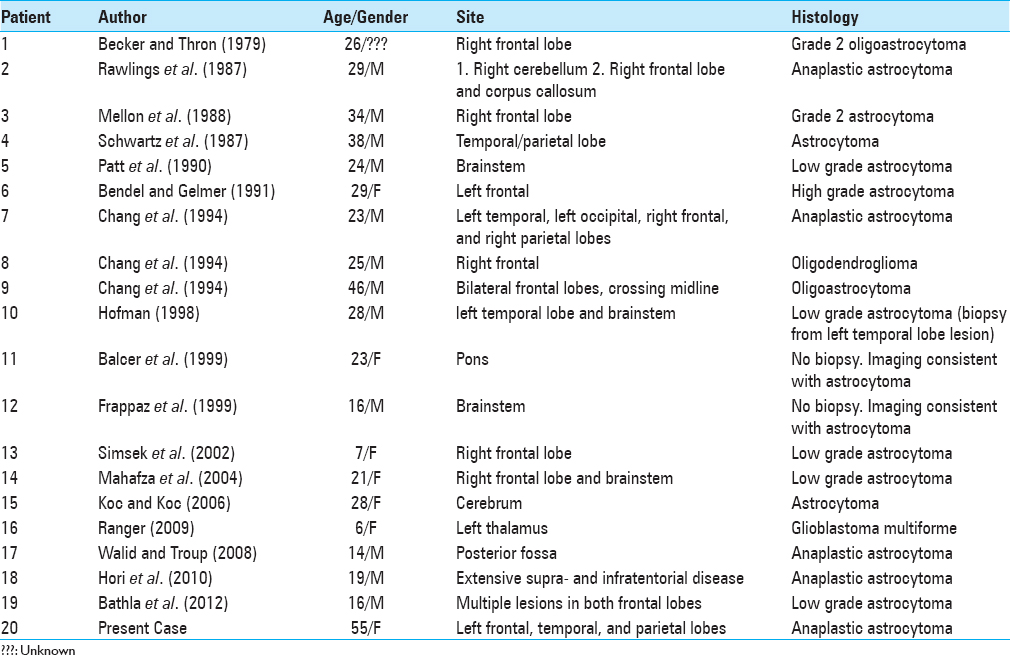
Intracranial lesions have been described previously and the most prevalent type of CNS tumors associated with Ollier disease is astrocytoma (low-grade to glioblastoma multiforme) and oligodendroglioma.[
CASE DESCRIPTION
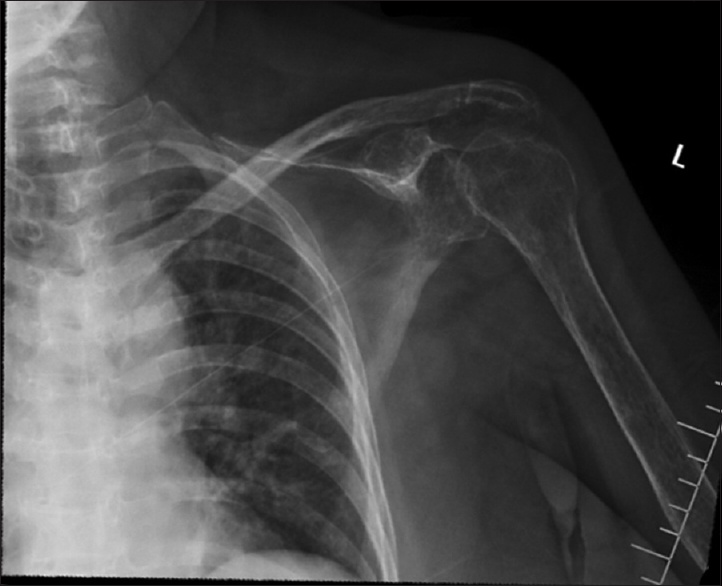
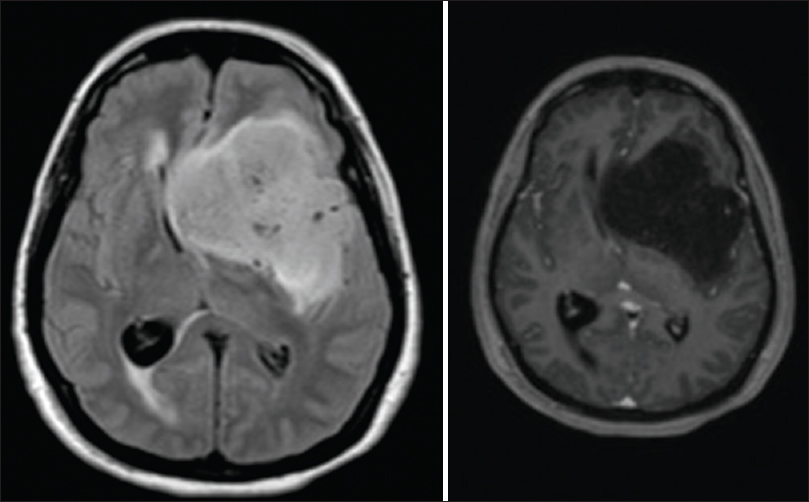
A 55-year-old woman with Ollier disease and congenital limb deformities presented to our clinic in October 2013. She was originally diagnosed in April 2011 with progressive right hemiparesis and language and memory problems. Imaging studies demonstrated a large left-sided hemispheric mass [
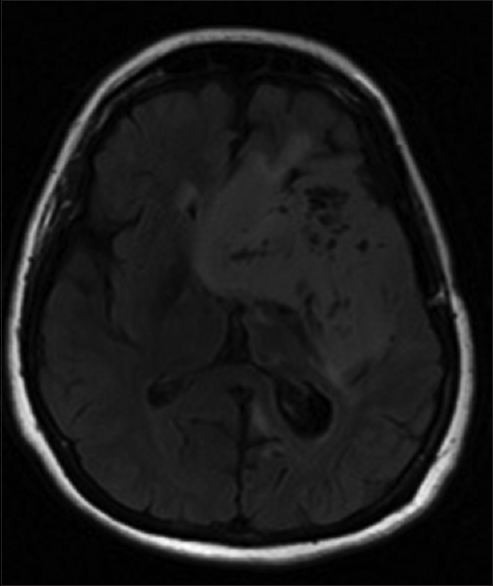
She was recently seen in the clinic in September 2014 and was remarkably stable despite the lack of any postoperative adjuvant therapy. On examination, she continues to have right facial droop and right hemiparesis but with intact cranial nerves II–XII. Left arm was immobile due to pain but the left leg had full strength. The patient had moderate expressive aphasia but followed requests and answered simple questions easily. There were no spasticity or contractures present. Psychiatric evaluation showed a pleasant, cooperative individual, with poor short-term memory and psychomotor slowing. The husband described typical hypothalamic gelastic seizures episodes, 2–3 brief episodes per month despite the fact that she was on seizure prophylaxis medication. She had an magnetic resonance imaging (MRI) in August 2014 that showed stable disease without any interval [
DISCUSSION
A retrospective analysis published by Bathla et al. shows cases of Ollier disease with gliomas diagnosed at a mean age of 23.7 years.[
Interestingly Bathla reported that 50% of the patients had a distinct frontal lobe lesion, followed by 37.5% with brainstem lesions.[
A potential explanation could be a difference in the tumor genotype; in patients with anaplastic astrocytomas without any association with Ollier Disease, isocitrate dehydrogense 1 (IDH1) has been demonstrated to be the single, most prominent prognostic factor for survival, followed by age, diagnosis, and methylguanine-DNA-methyltransferase (MGMT) status.[
These genetic variations have been correlated with outcome and the sequence from most to least favorable prognosis is as follows: (1) Anaplastic astrocytoma with IDH1 mutation, (2) glioblastoma with IDH1 mutation, (3) anaplastic astrocytoma without IDH1 mutation, and (4) glioblastoma without IDH1 mutation.[
CONCLUSION
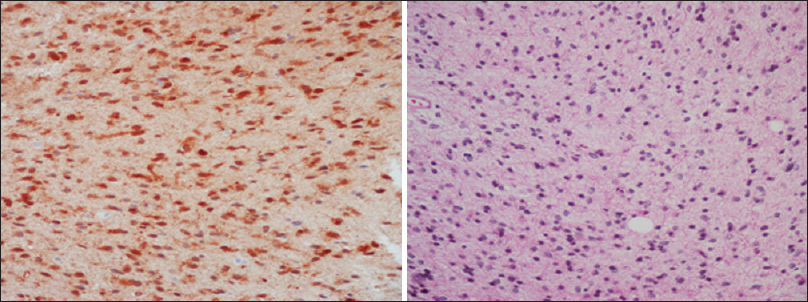
We present the case of a patient with Ollier disease and anaplastic astrocytoma who had a very favorable natural history of the CNS malignancy despite the lack of any adjuvant treatment after subtotal tumor resection. Analysis for IDH1 gene mutations in this patient was positive and IDH1 mutation in the tumor cells compared to wild type seems to have some protective effects in Ollier disease patients with anaplastic astrocytoma. An early genetic analysis after diagnosis can help in the prognostication of the illness and help the patients and their families to realistically deal with such a life-threatening cancer.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Amary M. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central periosteal chondromas but not in the other mesenchymal tumors. J Pathol. 2011. 224: 334-43
2. Amary M. Ollier disease and Maffucci syndrome are caused by somatic mosaic mutations of IDH1 and IDH2. Nat Genet. 2011. 43: 1262-5
3. Bathla G, Gupta S, Ong CK. Multifocal intracranial astrocytoma in a pediatric patient with Ollier disease. Indian J Radiol Imaging. 2012. 22: 58-62
4. Couvineau A, Wouters V, Bertrand G, Rouyer C, Gerard B, Boon LM. PTHR1 mutations associated with Ollier disease result in receptor loss of function. Hum Mol Genet. 2008. 17: 2766-75
5. D’Angelo L, Massimi L, Narducci A, Di Rocco C. Ollier disease. Childs Nerv Syst. 2009. 25: 647-53
6. Elens I, De Vleeschouwer S, Pauwels F, Van Gool S. Resection and Immunotherapy for Recurrent Grade III Glioma. ISRN Immunol 2012. 2011. p. 9-
7. Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A. Malignant astrocytic glioma: Genetics, biology, and paths to treatment. Genes Dev. 2007. 21: 2683-710
8. Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: Implications for classification of gliomas. Acta Neuropathol. 2010. 120: 707-18
9. Hori K, Matsumine A, Niimi R, Maeda M, Uchida K, Nakamura T. Diffuse Gliomas in an adolescent with multiple enchondromatosis (Ollier's disease). Oncol Lett. 2010. 1: 595-7
10. Kloosterhof NK, Bralten LB, Dubbink HJ, French PJ, van den Bent MJ. Isocitrate dehydrogenase-1 mutation: A fundamentally new understanding of diffuse glioma?. Lancet Oncol. 2011. 12: 83-91
11. Liu XY, Gerges N, Korshunov A, Sabha N, Khuong-Quang DA, Fontebasso AM. Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol. 2012. 124: 615-25
12. Ozisik YY, Meloni AM, Spanier SS, Bush CH, Kingsley KL, Sandberg AA. Deletion 1p in a low-grade chondrosarcoma in a patient with Ollier disease. Cancer Genet Cytogenet. 1998. 105: 128-33
13. Polivka J, Polivka J, Rohan V, Pesta M, Repik T, Pitule P. Isocitrate Dehydrogenase-1 Mutations as Prognostic Biomarker in Glioblastoma Multiforme Patients in West Bohemia. Biomed Res Int 2014. 2014. p. 735659-
14. Rozeman LB, Hameetman L, van Wezel T, Taminiau AH, Cleton-Jansen AM, Hogendoorn PC. cDNA expression profiling of chondrosarcomas: Ollier disease resembles solitary tumours and alteration in genes coding for components of energy metabolism occurs with increasing grade. J Pathol. 2005. 207: 61-71
15. Rynearson AL, Schowalter KA, Fink SR, Kollmeyer TM, Halder C, Sarkar G. Abstract 4207: The germline rs55705857 risk allele is not preferentially gained in gliomas with 8q24 duplication. Cancer Research. 2013. 73: 4207-
16. Silve C, Juppner H. Ollier disease. Orphanet J Rare Dis. 2006. 1: 37-






angelique monge
Posted April 8, 2021, 10:07 am
my brother was diagnosed with Ollier’s Disease when he was 5 yo (approximately 1972). He was diagnosed with a malignant brain tumor (glioblastoma) when he was 17 yo (approximately 1984). He passed away at the age of 23 yo (1990).
I read this paper and thought his case may help your cause.