- Department of Neurosurgery, National Defense Medical College, Tokorozawa, Saitama, Japan
- Department of Anatomy and Neurobiology, National Defense Medical College, Tokorozawa, Saitama, Japan
Correspondence Address:
Naoki Otani
Department of Neurosurgery, National Defense Medical College, Tokorozawa, Saitama, Japan
DOI:10.4103/2152-7806.185774
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Otani N, Wada K, Toyooka T, Fujii K, Kobayashi Y, Mori K. Operative surgical nuances of modified extradural temporopolar approach with mini-peeling of dura propria based on cadaveric anatomical study of lateral cavernous structures. Surg Neurol Int 07-Jul-2016;7:
How to cite this URL: Otani N, Wada K, Toyooka T, Fujii K, Kobayashi Y, Mori K. Operative surgical nuances of modified extradural temporopolar approach with mini-peeling of dura propria based on cadaveric anatomical study of lateral cavernous structures. Surg Neurol Int 07-Jul-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/operative-surgical-nuances-modified-extradural-temporopolar-approach-mini%e2%80%91peeling-dura-propria-based-cadaveric-anatomical-study-lateral-cavernous-structures/
Abstract
Background:Extradural temporopolar approach (ETA) has been modified as less invasive manner and named as trans-superior orbital fissure (SOF) approach with mini-peeling technique. The present study discusses the operative nuances of this modified technique on the basis of cadaveric study of lateral cavernous structures.
Methods:In five consecutive cadaveric specimens, we performed an extradural anterior clinoidectomy with mini-peeling of the dura propria to expose the anterior clinoid process entirely. We also investigated the histological characteristics of the lateral cavernous sinus (CS) between the dura propria and periosteal dura at the SOF, foramen rotundum (FR), and foramen ovale (FO) levels, and of each trigeminal nerve division.
Results:Coronal histological examination of the lateral wall of the CS showed invagination of the dura propria and periosteal dura into the SOF. In contrast, no such invagination was observed at the levels of the FR and FO. This finding supports the technical rationale of the only skeletonization of the SOF for peeling of the dura propria but not FR. In addition, our modified ETA method needs only minimal dural incision between the SOF and FR where no cranial nerves are present.
Conclusion:Our technical modification of ETA may be recommended for surgical treatment of paraclinoid lesions to reduce the risk of intraoperative neurovascular injury.
Keywords: Anterior clinoidectomy, cavernous sinus, extradural temporopolar approach, paraclinoid lesion, skull base surgery
INTRODUCTION
Extradural temporopolar approach (ETA), which requires peeling of dura propria of the temporal lobe dura mater from the lateral wall of the cavernous sinus (CS) and extradural anterior clinoidectomy (EAC), is a variant of the Dolenc's technique but focuses more on temporal lobe retraction over the dura mater and provides a surgical corridor to the central skull base via the opened CS.[
In contrast, ETA was recently modified to provide less invasive but adequate exposure of the anterior clinoid process (ACP)[
MATERIALS AND METHODS
Surgical procedure of extradural anterior clinoidectomy with mini-peeling
EAC with mini-peeling of dura propria was peformed as follows. A semi-coronal skin incision is performed followed by inter-fascial dissection. A standard frontotemporal craniotomy is performed up to the supra-orbital notch, and the temporal squama is rongeured out until the floor of the middle cranial fossa is exposed. The lesser wing of the sphenoid bone is flattened until the meningo-orbital band (MOB) is fully exposed. The middle fossa dura is dissected until the SOF, and the FR are exposed [
Figure 1
After standard frontotemporal craniotomy, the middle fossa dura is dissected until the foramen rotundum, and superior orbital fissure are exposed (a). Skeletonization of the foramen rotundum is not needed because this junction is naturally exposed at the foramen rotundum (a, arrow). The roof of the superior orbital fissure is skeletonized and opened to expose the junction between the dura propria of the temporal lobe and the periosteal dura (b, arrowheads). The bone around the meningo-orbital band is drilled and incised to a length of 4 mm (c). Peeling of the dura propria is started from the foramen rotundum to the lateral wall of the superior orbital fissure (d). V2: Second division of the trigeminal nerve
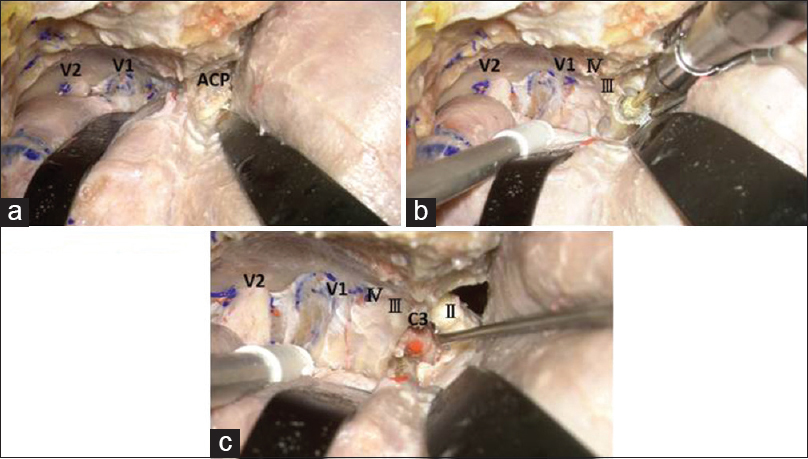
Figure 2
The dura propria is peeled from the lateral wall of the superior orbital fissure until the anterior clinoid process is exposed epidurally (a). Drilling of the anterior clinoid process with a high-speed drill using saline irrigation is started from the lateral part of the anterior clinoid process (b). After removal of the anterior clinoid process, the clinoid segment (C3) of the internal carotid artery can be seen (c). II: Optic nerve, III: Oculomotor nerve, IV: Trochlear nerve, V1: First division of the trigeminal nerve, V2: Second division of the trigeminal nerve
Cadaveric study
Specimen preparation
All cadavers were donated with written permission for anatomical study on the normal structures of the human body. The cadavers were initially fixed with a solution containing 3.3% formalin and 66% ethanol by perfusion through a femoral artery and underwent immersion fixation in the same fixative for several weeks. Cranial blood vessels were injected with colored silicone.
Cadaveric dissection
Two specimens were used for cadaveric dissection of ETA using a surgical microscope (Carl Zeiss Contravas; Zeiss, Inc., Montpelier, MD, USA) under ×4 to ×40 magnifications. All procedures were consistent with the clinical setting, and all observations were made from the surgeon's angle of view. All photographs illustrating anatomical structures are from the left side of the patient. Each head was fixed in a Mayfield head clamp and positioned for a standard approach. The bone dissections were performed with a Midas Rex drill (Midas Rex Institute, Fort Worth, TX, USA). The surgical technique of EAC with mini-peeling of the dura propria was performed and the anatomic features of the paraclinoid space were examined in stepwise dissections [Figures
Histological investigation
Five specimens were used for histological examination of the spatial relationship of the dural layers of the lateral wall of CS. The CS region including the SOF, FR, and foramen ovale (FO) was removed en bloc [
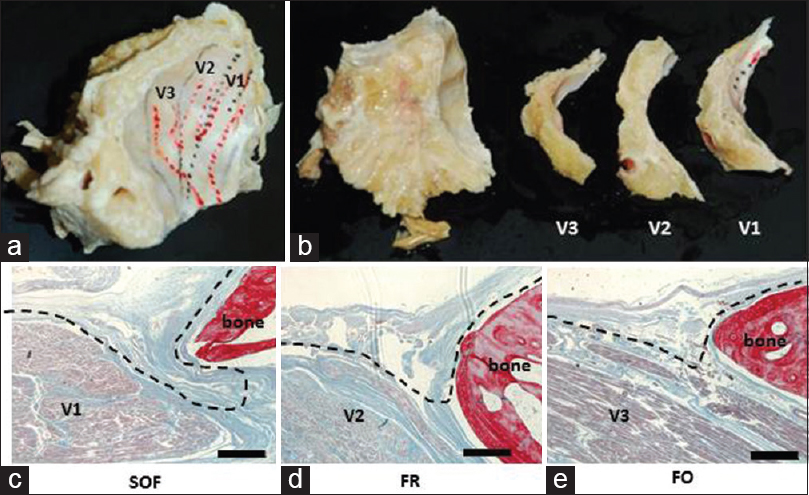
Figure 3
Cavernous sinus region including the superior orbital fissure, foramen rotundum, and foramen ovale was removed en bloc (a), and embedded in paraffin (b). The dura propria, which consists of the cranial nerve perineurium, is invaginated at the level of the superior orbital fissure (c). In contrast, no such invagination was observed at the levels of the foramen rotundum (d) and foramen ovale (e). Black broken line shows the layer of the peeling. V1: First division of the trigeminal nerve, V2: Second division of the trigeminal nerve, V3: Third division of the trigeminal nerve. Scale bars = 500 μm
RESULTS
Coronal histological sections of the lateral wall of CS showed that the two dural layers (dura propria and periosteal dura) were separate at the levels of SOF [
DISCUSSION
Removal of ACP[
Our schematic illustration of the dura propria of the temporal lobe and other anatomical structures is based on our cadaveric study and surgical experience [
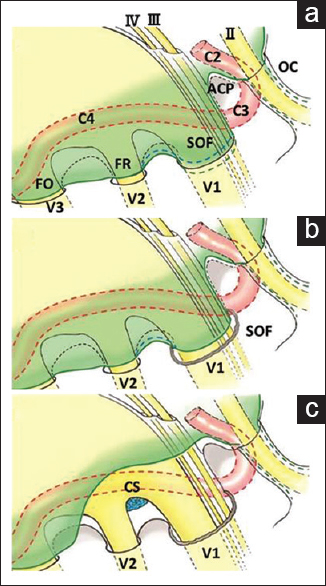
Figure 4
Schematic illustration of the dura propria of the temporal lobe and other anatomical structures. The junction between the dura propria and the periosteal dura was invaginated at the superior orbital fissure (a, blue broken line). Our method needs only minimal dural incision between the superior orbital fissure and foramen rotundum where no cranial nerves are present (b, blue broken line). The dura propria peeling can be performed until the anterior clinoid process is totally exposed epidurally (c). OC: Optic canal, II: Optic nerve, III: Oculomotor nerve, IV: Trochlear nerve
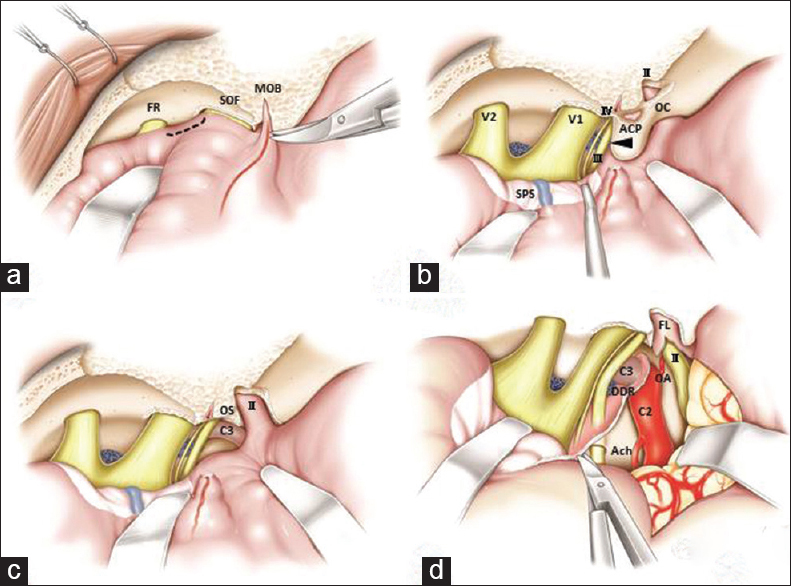
Figure 5
Schematic illustration of the operative techniques of extradural anterior clinoidectomy with mini-peeling of the dura propria followed by extradural temporopolar approach. During cutting of the meningo-orbital band, the tip of the micro-scissors is pointed to the exposed dura propria junction at the skeletonized superior orbital fissure (a). The dura propria incision can be limited to the dura propria between superior orbital fissure and foramen rotundum where no neurovascular structures exist (a, black broken line). The dura propria should be carefully peeled from the superior orbital fissure to preserve the sphenoparietal sinus on the peeled dura propria, which appears whitish until the anterior clinoid process is entirely exposed (b). Before drilling, the inferolateral part of the anterior clinoid process should be dissected because the extradural part of the oculomotor nerve passes there (b, arrow). After removal of the anterior clinoid process, the optic canal should be opened using a micro-punch. The clinoid segment (C3) of the internal carotid artery can be seen and the optic strut between the C3 and optic sheath is removed if necessary (c). Final operative view of the extradural temporopolar approach (d) suggests that incision of the falciform ligament and distal dural ring facilitates mobilization of the optic nerve and internal carotid artery, which contributes to a wide operative field in the infraoptic and subchiasmatic spaces without intraoperative neurovascular injury. OC: Optic canal, OS: Optic strut, II: Optic nerve, III: Oculomotor nerve, IV: Trochlear nerve, V1: First division of the trigeminal nerve, V2: Second division of the trigeminal nerve, SPS: Sphenoparietal sinus, DDR: Distal dural ring, FL: Falciform ligament, OA: Ophthalmic artery, Ach: Anterior choroidal artery
CONCLUSION
EAC requires extensive knowledge of the detailed anatomical structure of the SOF, MOB, and walls of the CS. On the basis of current cadaveric study of lateral cavernous structures, our modified extradural method requires skeletonization of SOF to expose interleticular layer and needs only minimal dural incision between SOF and FR where no cranial nerves are present. We recommend its less invasive, safe, and useful technique to entirely expose the ACP followed by modified ETA.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We give special thanks to Ms. Mayumi Watanabe for histological processing, and to Mr. Tohru Tamai, Mr. Hiroshi Sasaki, and Dr. Toshiyasu Matsui for cadaveric preparation.
References
1. Coscarella E, Baskaya MK, Morcos JJ. An alternative extradural exposure to the anterior clinoid process: The superior orbital fissure as a surgical corridor. Neurosurgery. 2003. 53: 162-6
2. Day AL. Aneurysms of the ophthalmic segment. A clinical and anatomical analysis. J Neurosurg. 1990. 72: 677-91
3. Day JD, Fukushima T, Giannotta SL. Cranial base approaches to posterior circulation aneurysms. J Neurosurg. 1997. 87: 544-54
4. Day JD, Giannotta SL, Fukushima T. Extradural temporopolar approach to lesions of the upper basilar artery and infrachiasmatic region. J Neurosurg. 1994. 81: 230-5
5. Dolenc VV. A combined epi- and subdural direct approach to carotid-ophthalmic artery aneurysms. J Neurosurg. 1985. 62: 667-72
6. Mori K, Yamamoto T, Nakao Y, Esaki T. Surgical simulation of extradural anterior clinoidectomy through the trans-superior orbital fissure approach using a dissectable three-dimensional skull base model with artificial cavernous sinus. Skull Base. 2010. 20: 229-35
7. Mori K.editors. Extradural temporopolar approach: Operative technique and application to aneurysm clipping surgery. Essential Practice of Neurosurgery. Nyon, Vaud Switzerland: World Federation of Neurosurgical Societies; 2013. p. 1614-23
8. Mori k. Orbitozygomatic approach and extradural temporopolar approach: Operative techniques and tips. Jpn J Neurosurg (Tokyo). 2014. 23: 785-93
9. Noguchi A, Balasingam V, Shiokawa Y, McMenomey SO, Delashaw JB. Extradural anterior clinoidectomy. Technical note. J Neurosurg. 2005. 102: 945-50
10. Nutik SL. Removal of the anterior clinoid process for exposure of the proximal intracranial carotid artery. J Neurosurg. 1988. 69: 529-34
11. Otani N, Wada K, Kobayashi Y, Kumagai K, Ueno H, Osada H. Extradural temporopolar approach for surgical management of paraclinoid lesions. No Shinkei Geka. 2014. 42: 907-16
12. Yonekawa Y, Ogata N, Imhof HG, Olivecrona M, Strommer K, Kwak TE. Selective extradural anterior clinoidectomy for supra- and parasellar processes. Technical note. J Neurosurg. 1997. 87: 636-42
13. Yoon BH, Kim HK, Park MS, Kim SM, Chung SY, Lanzino G. Meningeal layers around anterior clinoid process as a delicate area in extradural anterior clinoidectomy: Anatomical and clinical study. J Korean Neurosurg Soc. 2012. 52: 391-5