- Department of Chemistry, Rice University, Houston, Texas, USA
- The NanoCarbon Center, Rice University, Houston, Texas, USA
- Department of Material Science and Nanoengineering, Rice University, Houston, Texas, USA
Correspondence Address:
James M. Tour
Department of Chemistry, Rice University, Houston, Texas, USA
The NanoCarbon Center, Rice University, Houston, Texas, USA
Department of Material Science and Nanoengineering, Rice University, Houston, Texas, USA
DOI:10.4103/sni.sni_361_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: William K. A. Sikkema, Andrew B. Metzger, Tuo Wang, James M. Tour. Physical and electrical characterization of TexasPEG: An electrically conductive neuronal scaffold. 26-May-2017;8:84
How to cite this URL: William K. A. Sikkema, Andrew B. Metzger, Tuo Wang, James M. Tour. Physical and electrical characterization of TexasPEG: An electrically conductive neuronal scaffold. 26-May-2017;8:84. Available from: http://surgicalneurologyint.com/surgicalint-articles/physical-and-electrical-characterization-of-texaspeg-an-electrically-conductive-neuronal-scaffold/
Abstract
Background:Graphene and its derivatives have been shown to be biocompatible and electrically active materials upon which neurons readily grow. The fusogen poly(ethylene glycol) (PEG) has been shown to improve outcomes after cervical and dorsal spinal cord transection. The long and narrow PEGylated graphene nanoribbon stacks (PEG-GNRs) with their 5 μm × 200 nm × 10 nm dimensions can provide a scaffold upon which neurons can grow and fuse. We disclose here the extensive characterization data for the PEG-GNRs.
Methods:PEG-GNRs were chemically synthesized and chemically and electrically characterized.
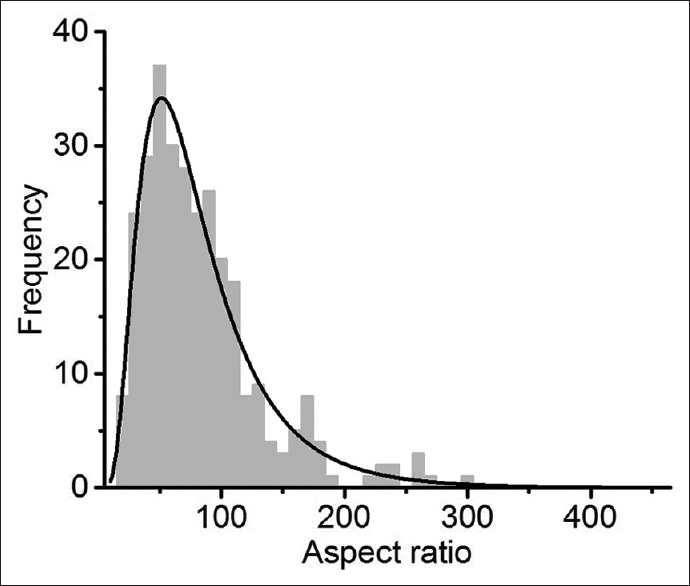
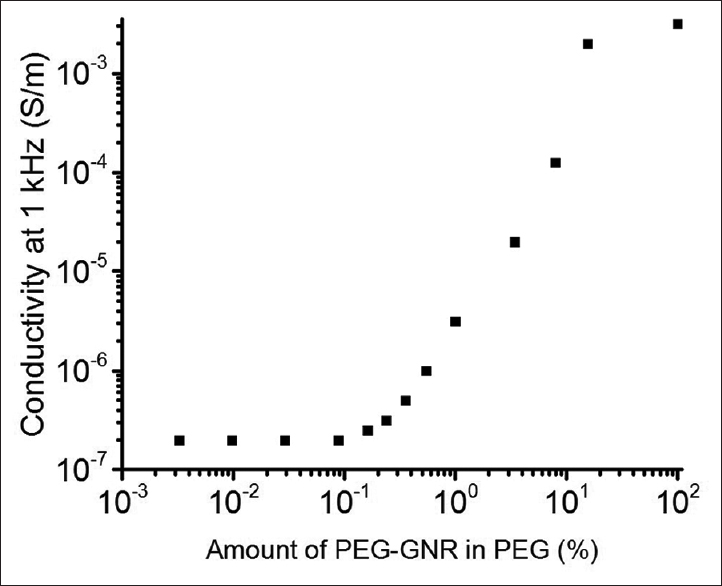
Results:The average aspect ratio of the PEG-GNRs was determined to be ~85, which corresponds to a critical percolation value (the point where insulating material becomes conductive by addition of conductive particles) of 1%. However, there was not a sharp increase in AC conductivity at frequencies relevant to action potentials.
Conclusion:A robust characterization of PEG-GNRs is discussed, though the precise origin of efficacy in improving outcomes following spinal cord transection is not known.
Keywords: Fusogen, graphene, nanoribbons, PEG
INTRODUCTION
Graphitic structures have been shown to electrically stimulate, physically support, and organize the three-dimensional (3D) structure of neurons.[
Pure poly(ethylene glycol) (PEG) can restore at least partial motor function in rodents by acting as a fusogen to seal blunt ends of neuronal processes and connect neurons across the gap.[
Due to their large physical size, high molecular weight (~109 g/mol) and high aspect ratio, GNRs might remain in the tissue much longer. In addition, their conductive properties might allow them to act as an electrical conduit to restore conduction through the fusion interface much more quickly.[
This paper complements a sister paper[
MATERIALS AND METHODS
PEG-GNR (TexasPEG): Multi-walled carbon nanotubes (MWCNTs) were obtained from EMD Merck (produced by Mitsui & Co., lot no. 2699-64E) and were used as received. Tetrahydrofuran (THF) was dried over solid KOH for several days, degassed, and freshly distilled from sodium/benzophenone under a N2 atmosphere. All chemicals were purchased from Sigma-Aldrich unless otherwise specified. Thermogravimetric analysis (TGA) measurements were performed on a TA instruments Q-600 Simultaneous TGA/DSC. The temperature was ramped at 10°C/min until 850°C under argon. For transmission electron microscopy (TEM, JEOL JEM 2100F) analysis, the PEG-GNRs were dispersed in water and drop cast onto a lacey carbon grid and allowed to dry for 6 h. For scanning electron microscope (SEM) analysis, the PEG-GNRs were dispersed in o-dichlorobenzene, briefly sonicated in a bath sonicator, and deposited on a silicon wafer at an approximate density of 1 PEG-GNR per 500 μm2, from which the solvent was evaporated on a heat plate at <100°C. The sample was imaged by an FEI Quanta 400 ESEM FEG instrument. Thirty images were taken in a direct line across the sample starting from a random location to minimize selection bias, and the resulting images were analysed with the aid of ImageJ. Conductivity measurements were performed with a home-built copper parallel plate dip-probe connected to a Hewlett-Packard 3577a Network Analyzer. Calibrations were performed using methanol and a short-circuit liquid metal standard.
1.0 g of Mitsui MWCNTs was added to a 1 L oven-dried, nitrogen-purged, Schlenk flask; 500 mL of THF was added. 2.5 mL of eutectic NaK (1:3.3 by mass, 1:1.9 by mol) was added under nitrogen. The reaction mixture was stirred at room temperature for 3 days, until very few liquid droplets of NaK remained. The reaction was cooled in a dry ice/acetone bath to −78°C, and 30 g (0.7 mol) of gaseous ethylene oxide was added from a lecture bottle over 90 min. The mixture was slowly brought to room temperature and stirred for 3 days. A mixture of NaH (20 mmol, 0.53 g) and propargyl bromide (20 mmol, 2.4 g) suspended/dissolved in dry toluene was added to terminate the ethylene oxide polymerization. The reaction was quenched by adding 20 L of water, and the dark gray precipitate was collected via filtration on a 0.22 μm polyethersulfone (PES) membrane. The dark gray precipitate was filtered through a polytetrafluoroethylene (PTFE) membrane (0.45 μm), followed by crossflow filtration with a 50 kDa MWCO PES filter to remove unbound polymer. The PEG-GNRs final product (1.3 g) was collected on a PTFE membrane (0.45 μm), washed with DI water (3 × 100 mL), ethanol (3 × 100 mL), DI water (3 × 100 mL), and dried under high vacuum overnight. The propargyl units were added to some of the termini for future peptide additions if desired. Before use, the PEG-GNRs were dispersed in PEG 600 with an IKA T25 digital Ultra-Turrax machine running at 1000 rpm with an S25N-18G Dispersing element attachment. (0.5–1% (w/v) by GNR concentration). The mixture was tightly sealed in a 50 mL conical vial and was sterilized by 120°C pressurized steam for 30 min.
RESULTS AND DISCUSSION
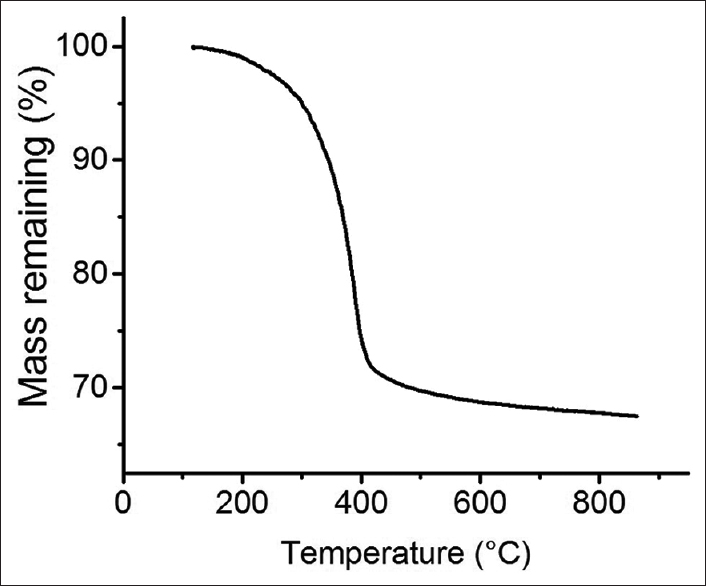
Characterization of relevant physical and electrical properties was performed. First, to assess the amount of polymer covalently bound to the GNRs, TGA was performed. PEG decomposes fully by 400°C, while the GNRs are stable under the temperatures tested. The PEG-GNRs are composed of 30% PEG, while GNRs comprise the remaining 70% [
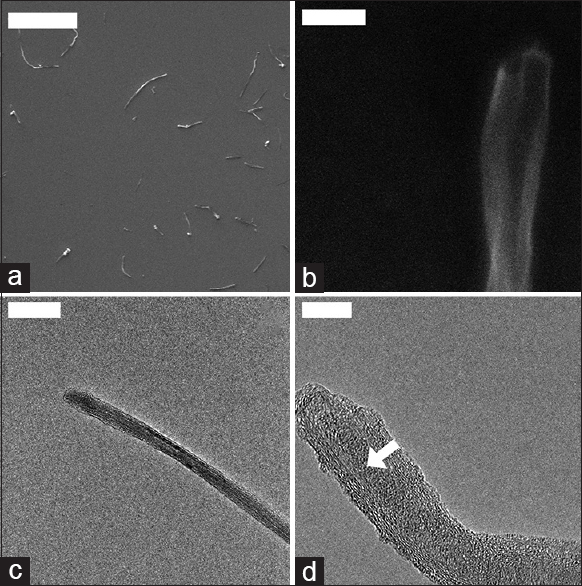
By examination of the SEM and TEM images in
Figure 2
SEM and TEM analysis revealed the ribbon-like structure of the PEG-GNRs. (a) Individualized graphene nanoribbon stacks. Scale = 10 μm. (b) Open end of a large GNR structure. Scale = 200 nm; (c) End of thin GNR. Scale = 100 nm; (d) floppy end of a GNR stack, showing the triangular stack of GNRs. The white arrow shows the stack increasing in thickness. Scale = 10 nm
The critical percolation concentration is determined by the aspect ratio of the conductive structures. This was calculated by measuring the lengths of individually dispersed PEG-GNRs on a silicon substrate of every PEG-GNR longer than 1 μm [
The percolation conductivity was measured from a concentration of 0.003% to 100% of PEG-GNRs in PEG at 1 kHz, as this is approximately the frequency of neuronal signals [
CONCLUSION
In the sister paper,[
Financial support and sponsorship
We thank the Air Force Office of Scientific Research (FA9550-14-1-0111) for support.
Conflicts of interest
There are no conflicts of interest.
References
1. Akhavan O, Ghaderi E. Differentiation of human neural stem cells into neural networks on graphene nanogrids. J Mater Chem B. 2013. 1: 6291-301
2. Akhavan O, Ghaderi E, Abouei E, Hatamie S, Ghasemi E. Accelerated differentiation of neural stem cells into neurons on ginseng-reduced graphene oxide sheets. Carbon. 2014. 66: 395-406
3. Bitner BR, Marcano DC, Berlin JM, Fabian RH, Cherian L, Culver JC. Antioxidant carbon particles improve cerebrovascular dysfunction following traumatic brain injury. J Neurotrauma. 2013. 30: 786-96
4. Bornhoeft LR, Castillo AC, Smalley PR, Kittrell C, James DK, Brinson BE. Teslaphoresis of carbon nanotubes. ACS Nano. 2016. 10: 4873-81
5. Canavero S. The “Gemini” spinal cord fusion protocol: Reloaded. Surg Neurol Int. 2015. 6: 18-
6. Fabbro A, Scaini D, Leon V, Vázquez E, Cellot G, Privitera G. Graphene-based interfaces do not alter target nerve cells. ACS Nano. 2015. 10: 615-23
7. Fabbro A, Sucapane A, Toma FM, Calura E, Rizzetto L, Carrieri C. Adhesion to carbon nanotube conductive scaffolds forces action-potential appearance in immature rat spinal neurons. PLoS One. 2013. 8: 1-14
8. Garboczi EJ, Snyder KA, Douglas JF, Thorpe MF. Geometrical percolation threshold of overlapping ellipsoids. Phys Rev E. 1995. 52: 819-28
9. Kim C, Sikkema WKA, Hwang I, Oh H, Kim UJ. Spinal cord fusion with PEG-GNRs (TexasPEG): neurophysiological recovery in 24 hours in rats. Surg Neurol Int. 2016. 7: S632-6
10. Kim CY. PEG-assisted reconstruction of the cervical spinal cord in rats: Effects on motor conduction at 1 h. Spinal Cord. 2016. 1: 1-3
11. Kim J, Park S, Kim YJ, Jeon CS, Lim KT, Seonwoo H. Monolayer graphene-directed growth and neuronal differentiation OF mesenchymal stem cells. J Biomed Nanotechnol. 2015. 11: 2024-33
12. Luo J, Borgens R, Shi R. Polyethylene glycol immediately repairs neuronal membranes and inhibits free radical production after acute spinal cord injury. J Neurochem. 2002. 83: 471-80
13. Marcano DC, Bitner BR, Berlin JM, Jarjour J, Lee JM, Jacob A. Design of poly(ethylene glycol)-functionalized hydrophilic carbon clusters for targeted therapy of cerebrovascular dysfunction in mild traumatic brain injury. J Neurotrauma. 2012. 8: 1-8
14. Nilewski LG, Sikkema WKA, Tour JM, Kent TA. Carbon nanoparticles and oxidative stress: Could an injection stop brain damage in minutes?. Nanomedicine. 2015. 10: 1677-9
15. Sahni D, Jea A, Mata JA, Marcano DC, Sivaganesan A, Berlin JM. Biocompatibility of pristine graphene for neuronal interface. J Neurosurg Pediatr. 2013. 11: 575-83
16. Samuel ELG, Marcano DC, Berka V, Bitner BR, Wu G, Potter A. Highly efficient conversion of superoxide to oxygen using hydrophilic carbon clusters. Proc Natl Acad Sci. 2015. 112: 2343-8
17. Wang Z. Alignment of graphene nanoribbons by an electric field. Carbon N Y. 2009. 47: 3050-3
18. Xu W, Lee T-W. Recent progress in fabrication techniques of graphene nanoribbons. Mater Horiz. 2016. 3: 186-207
19. Zhou K, Thouas GA, Bernard CC, Nisbet DR, Finkelstein DI, Li D. Method to impart electro- and biofunctionality to neural scaffolds using graphene-polyelectrolyte multilayers. ACS Appl Mater Interfaces. 2012. 4: 4524-31