- Endocrinology Division, Department of Clinical Medicine, Faculty of Medical Sciences, University of Campinas, Campinas, Brazil
- Oncology Division, Department of Clinical Medicine, Faculty of Medical Sciences, University of Campinas, Campinas, Brazil
- Department of Radiology, Faculty of Medical Sciences, University of Campinas, Campinas, Brazil
- Department of Pathology, Faculty of Medical Sciences, University of Campinas, Campinas, Brazil
- Department of Neurology, Faculty of Medical Sciences, University of Campinas, Campinas, Brazil
- Division of Nuclear Medicine, Department of Radiology, Faculty of Medical Sciences, University of Campinas, Campinas, Brazil
Correspondence Address:
Heraldo Mendes Garmes
Division of Nuclear Medicine, Department of Radiology, Faculty of Medical Sciences, University of Campinas, Campinas, Brazil
DOI:10.4103/sni.sni_498_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Heraldo Mendes Garmes, José Barreto Campello Carvalheira, Fabiano Reis, Luciano de Souza Queiroz, Mateus Dal Fabbro, Vanessa de Fatima Porto Souza, Allan de Oliviera Santos. Pituitary carcinoma: A case report and discussion of potential value of combined use of Ga-68 DOTATATE and F-18 FDG PET/CT scan to better choose therapy. 01-Aug-2017;8:162
How to cite this URL: Heraldo Mendes Garmes, José Barreto Campello Carvalheira, Fabiano Reis, Luciano de Souza Queiroz, Mateus Dal Fabbro, Vanessa de Fatima Porto Souza, Allan de Oliviera Santos. Pituitary carcinoma: A case report and discussion of potential value of combined use of Ga-68 DOTATATE and F-18 FDG PET/CT scan to better choose therapy. 01-Aug-2017;8:162. Available from: http://surgicalneurologyint.com/surgicalint-articles/pituitary-carcinoma-a-case-report-and-discussion-of-potential-value-of-combined-use-of-ga%e2%80%9168-dotatate-and-f%e2%80%9118-fdg-petct-scan-to-better-choose-therapy/
Abstract
Background:Pituitary carcinoma is extremely rare and carries a very poor prognosis. In most cases, apparently indolent tumors become malignant; however, there are no satisfactory biomarkers for predicting tumor behavior. Thus, scientific advances in the search for new biological markers, diagnostic methods, and therapies are needed to improve the prognosis of these patients.
Case Description:We report the case of a woman with initial diagnosis of nonfunctioning pituitary adenoma which evolved to carcinoma after 4 years. Diagnosis was confirmed after biopsy of metastatic pulmonary nodules, in which neoplastic cells were immunohistochemically positive for chromogranin, synaptotophysin, prolactin, and growth hormone. Investigation with conventional somatostatin receptor scintigraphy, positron emission tomography-computed tomography (PET-CT) with Ga-68 DOTATATE and F-18 fluorodeoxyglucose (FDG) are showed. During temozolomide therapy, our patient had severe pancytopenia resulting in death from generalized infection despite 10 days of intensive care.
Conclusion:The present case of an aggressive pituitary carcinoma rising from a typical adenoma illustrates the importance of developing new prognostic biomarkers in these cases. In addition to demonstrating a serious side effect with the use of temozolomide, our case report suggests that the combined use of Ga-68 DOTATATE and F-18 FDG PET-CT scan may scale somatostatin receptors vs. tumor aggressiveness, therefore, helping to better choose the therapy for aggressive pituitary tumors.
Keywords: Ga-68 DOTATATE PET/CT, pituitary carcinoma, temozolomide
INTRODUCTION
Pituitary carcinoma accounts for only 0.1% of all pituitary tumors, representing less than 200 cases in the English language literature. This entity is defined by a primary sellar tumor associated with noncontiguous intracranial lesions or metastasis to distant sites.[
In this report, the authors describe the case of a young woman with primary diagnosis of a nonfunctioning pituitary adenoma, which evolved into carcinoma after 4 years of follow-up.
Although several studies have demonstrated security with the use of temozolomide concurrent with radiation therapy,[
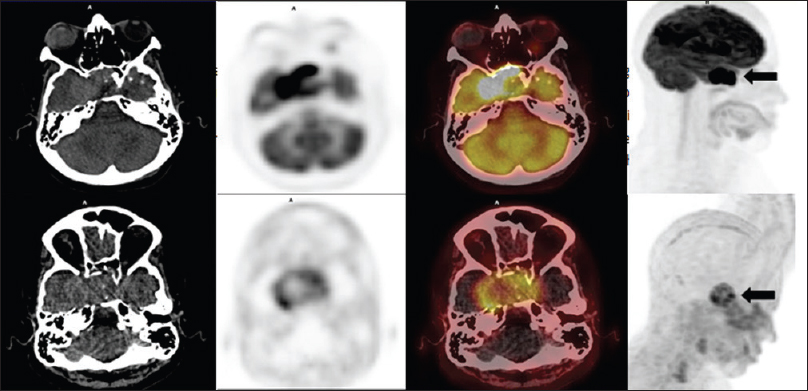
The investigation with conventional somatostatin receptor scintigraphy, positron emission tomography-computed tomography (PET-CT) with Ga-68 DOTATATE and F-18 fluorodeoxyglucose (FDG), treatment, and fatal outcome of this aggressive pituitary carcinoma are discussed.
CASE REPORT
A 32-year-old Caucasian woman was referred to the endocrinology department with 18-month history of right temporal headache, which was accompanied by visual acuity reduction as well as amenorrhea and galactorrhea. She also reported fatigue, weakness, nausea, and sporadic vomiting. Visual field evaluation demonstrated left eye amaurosis and temporal hemianopsia and lower nasal quadrantanopsia in the right eye. Hormonal evaluation was compatible with panhypopituitarism, and the patient was started on cortisol and T4 replacement therapy.
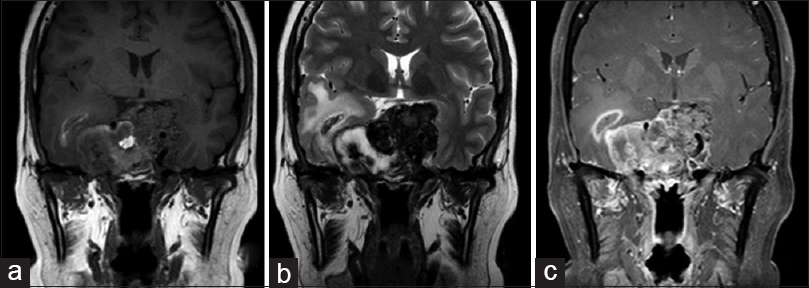
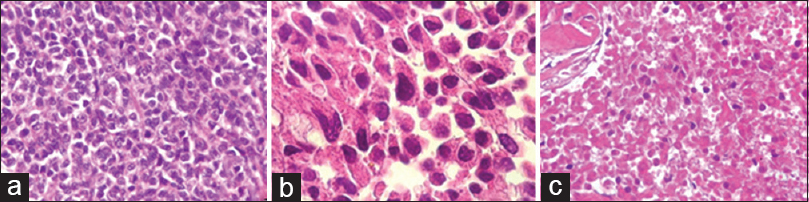
Magnetic resonance imaging (MRI) showed a 4.0 cm diameter solid lobulated lesion with sellar and suprasellar components, predominantly isointense on T1 and hypointense on T2-weighted images and extension to both the cavernous sinuses and optic chiasm. Given that the lesion was associated with optic tract compression, a transesfenoidal surgery for nonfunctioning pituitary macroadenoma was performed. The pathological evaluation revealed pituitary adenoma. Immunohistochemistry demonstrated that Ki-67 was positive in 3% of the cells and supporting the diagnosis of a canonical pituitary adenoma. In addition, p53 staining was positive in rare cells.
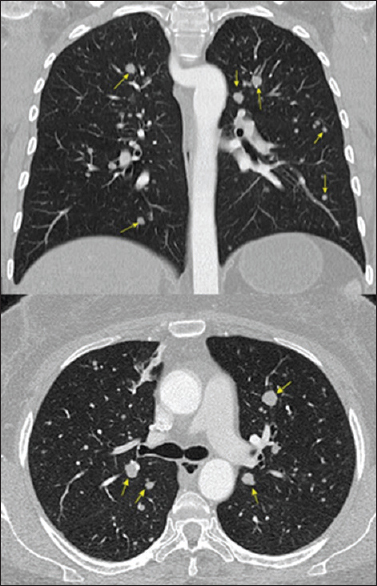
Although the headache persisted, the patient visual acuity significantly improved, as demonstrated by a striking improvement in visual field examination. The patient was started on cabergoline 0.5 mg 6 cps/week and remained free of progression for 4 years, when in a follow-up chest CT multiple pulmonary nodules with soft tissue density in both lung fields were detected, measuring 0.5–1.2 cm [
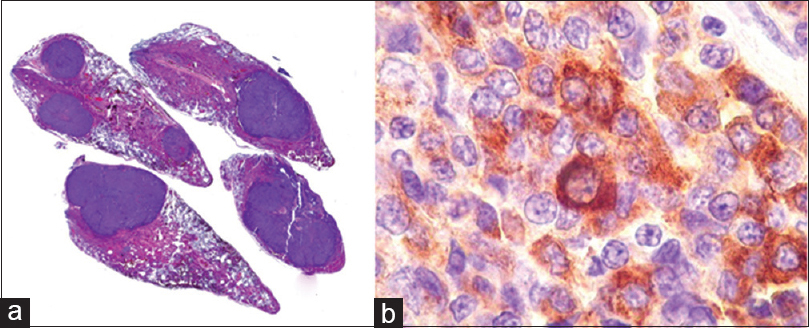
F-18 FDG images showed a markedly increased uptake in the pituitary (SUV 33) as well as in multiple lung nodules (SUV 11.1), pancreas (SUV 6), liver lesions (SUV 10.4) and right kidney nodule (SUV 8.5). Lung biopsy via bronchoscopy confirmed a moderately differentiated neuroendocrine tumor immunohistochemically positive for chromogranin, synaptophysin, prolactin, and growth hormone. Ki-67 was positive in approximately 10% of the cells [
Given the presence of a pancreatic lump, the diagnosis of a primary pancreatic neuroendocrine tumor was considered, however, the turcica sella MRI showed significantly increased tumor size and signs of chiasm and right optic nerve compression [
Tumor tissue of this second sample showed small neoplastic epithelial cells either cubic or polygonal, with slight to moderate nuclear pleomorphism, dense chromatin, and scant pink cytoplasm. The cells were arranged in dense blocks amid fibrous tissue, with extensive necrotic areas, which also showed phagocytosed hemossiderin granules and hematoidin crystals. Mitosis were rare. The diagnosis of pituitary adenocarcinoma was based on the previous lung biopsy which disclosed a metastatic neuroendocrine tumor. The extensive necrotic areas in the pituitary tumor also supported the diagnosis of malignancy [
After the diagnosis of pituitary carcinoma and based on PET-CT images obtained with F-18 FDG and Ga-68 DOTATATE, the oncology team indicated concomitant radiochemotherapy. The planned radiotherapy was a total of 5400 cGy (180 cGy × 30 days) plus temozolomide 75 mg/m2/day during the period of radiotherapy. However, on the 19th day of the treatment, the patient returned reporting of fatigue, sore throat, dry cough, and ecchymoses. Blood examination revealed pancytopenia, and despite intensive treatment, the patient died after 10 days due to generalized infection. A postmortem examination was not performed.
DISCUSSION
Because only 25% of the nonfunctioning macroadenomas increase in size significantly during follow-up[
Some pituitary tumors that will become carcinoma demonstrate their aggressive behavior early, recur quickly after surgical debulking, and progress rapidly to carcinoma; on the other hand, certain tumors progress to carcinoma after many years of follow-up.[
Even without significant growth of the tumor, the disease evolved with the emergence of metastases, showing the need for early markers of evolution in these cases. Histopathological diagnosis in the first surgery was pituitary adenoma, and there were no histological and imunohistochemical features of aggressiveness. Although some studies have shown a strong relationship between Ki-67 index and tumor aggressiveness (mean of 11.9 ± 3.4 for carcinomas to 1.4 ± 0.15 for adenomas),[
The presence of metastasis of a neuroendocrine tumor may indicate pituitary carcinoma, however, the presence of a pancreatic lump in our patient left a doubt regarding the possibility of a neuroendocrine tumor of the pancreas, hindering and delaying the diagnosis of pituitary carcinoma.
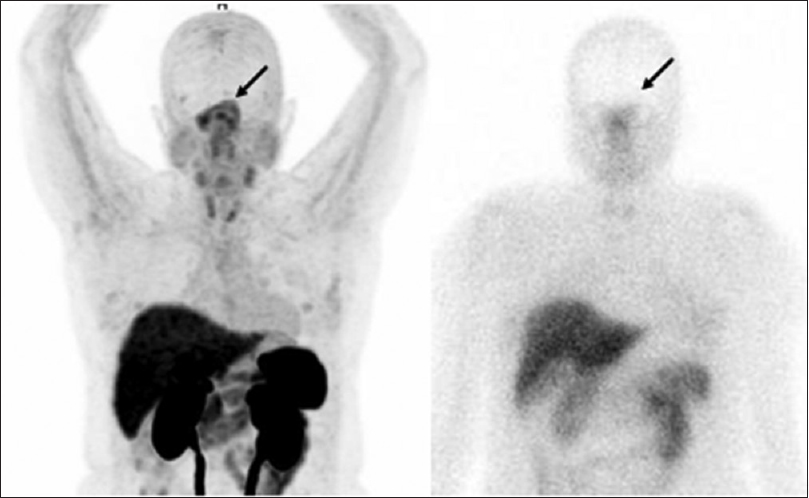
Whole body scintigraphy after Tc-99m HYNIC-octreotide injection was less sensitive to detect lesions with increased somatostatin receptors expression than three-dimensional PET-CT Ga-68 DOTATATE images in our patient, it can be verified that the former is more sensitive to detect somatostatin receptors expression. This greater sensitivity has already been described for neuroendocrine tumors;[
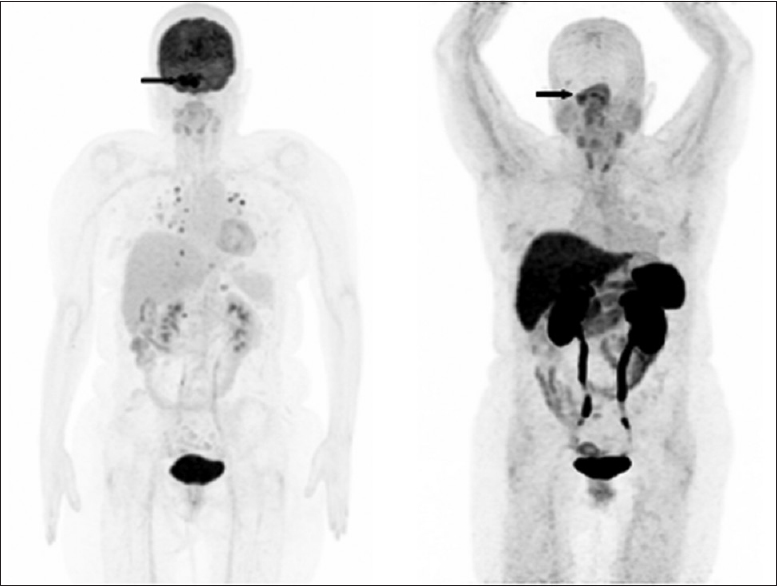
In our patient, the concomitant evaluation of PET-CT scan using F-18 FDG and Ga-68 DOTATATE showed an uptake almost eight times more intense with F-18 FDG. This different uptake is probably related with the high aggressiveness of the lesion. Carrying out these two examinations has the potential to define the aggressiveness of the tumor and thus help to indicate which is the better therapy, e.g., molecular-targeted therapy using somatostatin analogs and peptide receptor radionuclide therapy targeting somatostatin receptors or chemotherapy. A recent study showed that in a patient with a PET-CT scan showing multiple foci of increased Ga-68 DOTATATE uptake in pituitary and posterior fossa lesions, the tumor remained stable over 4 years after Lu-177 DOTATATE therapy.[
In contrast to a recently published study that showed high uptake of Ga-68 DOTATATE in a patient with pituitary carcinoma that the authors proposed Lu-177 DOTATATE therapy, the rational to use temozolomide in our patient was a lower uptake with Ga-68 DOTATATE associated with high F-18 FDG uptake compatible with tumoral aggressiveness.[
The prognosis for the patient of pituitary carcinoma is very poor. Most of the cases described in the current literature show rapid evolution after confirmation of metastasis, despite using aggressive treatments available.[
In conclusion, the present case of an aggressive pituitary carcinoma arising from a typical adenoma show how a benign disease can transform into a devastating one. Thus, the development of new prognostic biomarkers should be an imperative task to better control aggressive pituitary tumors. In addition to demonstrating a serious side effect with the use of temozolomide, our case report suggests that the combined use of Ga-68 DOTATATE and F-18 FDG PET/CT scan may scale somatostatin receptors vs. tumor aggressiveness, therefore helping to better choose the therapy for pituitary carcinoma.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA. Dose-dense temozolomide for newly diagnosed glioblastoma: A randomized phase III clinical trial. J Clin Oncol. 2013. 31: 4085-91
2. Karamouzis I, Berardelli R, Prencipe N, Berton A, Bona C, Stura G. Retrospective observational analysis of non-irradiated non-functioning pituitaryadenomas. J Endocrinol Invest. 2015. 38: 1191-7
3. Kovacs K. The 2004 WHO classification of pituitary tumors: Comments. Acta Neuropathol. 2006. 111: 62-3
4. Lim S, Shahinian H, Maya MM, Yong W, Heaney AP. Temozolomide: A novel treatment for pituitary carcinoma. Lancet Oncol. 2006. 7: 518-20
5. Losa M, Bogazzi F, Cannavo S, Ceccato F, Curtò L, De Marinis L. Temozolomide therapy in patients with aggressive pituitary adenomas or carcinomas. J Neurooncol. 2016. 126: 519-25
6. Love AP, Hinton DR, Krieger MD, Weiss MH. Invasive pituitary adenomas: Significance of proliferation parameters. Pituitary. 1999. 2: 117-22
7. Maclean J, Aldridge M, Bomanji J, Short S, Fersht N. Peptide receptor radionuclide therapy for aggressive atypical pituitary adenoma/carcinoma: Variable clinical response in preliminary evaluation. Pituitary. 2014. 17: 530-8
8. Mao Y, Yao Y, Zhang LW, Lu YC, Chen ZP, Zhang JM. Does Early Postsurgical Temozolomide Plus Concomitant Radiochemotherapy Regimen Have Any Benefit in Newly-diagnosed Glioblastoma Patients? A Multi-center, Randomized, Parallel, Open-label, Phase II Clinical Trial. Chin Med J. 2015. 128: 2751-8
9. Molitch ME. Pituitary tumours: Pituitary incidentalomas. Best Pract Res. 2009. 23: 667-75
10. Ntali G, Capatina C, Fazal-Sanderson V, Byrne JV, Cudlip S, Grossman A. Mortality in patients with non-functioning pituitary adenoma is increased: Systematic analysis of 546 cases with long follow-up. Eur J Endocrinol. 2016. 174: 137-45
11. Novruzov F, Aliyev JA, Jaunmuktane Z, Bomanji JB, Kayani I. The use of (68) Ga DOTATATE PET/CT for diagnostic assessment and monitoring of (177) Lu DOTATATE therapy in pituitary carcinoma. Clin Nucl Med. 2015. 40: 47-9
12. Oh S, Prasad V, Lee DS, Baum RP. Effect of Peptide Receptor Radionuclide Therapy on Somatostatin Receptor Status and Glucose Metabolism in Neuroendocrine Tumors: Intraindividual Comparison of Ga-68 DOTANOC PET/CT and F-18 FDG PET/CT. Int J Mol Imaging. 2011. 2011: 524130-
13. Ragel BT, Couldwell WT. Pituitary carcinoma: A review of the literature. Neurosurg Focus. 2004. 16: E7-
14. Roncaroli F, Scheithauer BW, Horvath E, Erickson D, Tam CK, Lloyd RV. Silent subtype 3 carcinoma of the pituitary: A case report. Neuropathol Appl Neurobiol. 2010. 36: 90-4
15. Salehi F, Agur A, Scheithauer BW, Kovacs K, Lloyd RV, Cusimano M. Ki-67 in pituitary neoplasms: A review—Part I. Neurosurgery. 2009. 65: 429-37
16. Sav A, Rotondo F, Syro LV, Di Ieva A, Cusimano MD, Kovacs K. Invasive, atypical and aggressive pituitary adenomas and carcinomas. Endocrinol Metab Clin North Am. 2015. 44: 99-104
17. Scheithauer BW, Kovacs KT, Laws ER, Randall RV. Pathology of invasive pituitary tumors with special reference to functional classification. J Neurosurg. 1986. 65: 733-44
18. Song Y, Qi S, Peng Y, Long H, Liu H. Non-functioning pituitary carcinoma: Report of two cases and review of literature. Nan Fang Yi Ke Da Xue Xue Bao. 2012. 32: 539-43
19. Thapar K, Kovacs K, Scheithauer BW, Stefaneanu L, Horvath , Pernicone PJ. Proliferative activity and pituitary adenomas and carcinomas among invasiveness: An analysis using the MIB-1 antibody. Neurosurgery. 1996. 38: 99-106
20. Xiao J, Zhu Z, Zhong D, Ma W, Wang R. Improvement in diagnosis of metastatic pituitary carcinoma by 68Ga DOTATATE PET/CT. Clin Nucl Med. 2015. 40: 129-31