- Department of Neurosurgery, Jena University Hospital, Friedrich Schiller University Jena, Germany
- Department of Neurology, Jena University Hospital, Friedrich Schiller University Jena, Germany
Correspondence Address:
Diaa Al Safatli
Department of Neurosurgery, Jena University Hospital, Friedrich Schiller University Jena, Germany
DOI:10.4103/sni.sni_479_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Diaa Al Safatli, Albrecht Guenther, Aaron Lawson McLean, Albrecht Waschke, Rolf Kalff, Christian Ewald. Prediction of 30-day mortality in spontaneous cerebellar hemorrhage. 20-Nov-2017;8:282
How to cite this URL: Diaa Al Safatli, Albrecht Guenther, Aaron Lawson McLean, Albrecht Waschke, Rolf Kalff, Christian Ewald. Prediction of 30-day mortality in spontaneous cerebellar hemorrhage. 20-Nov-2017;8:282. Available from: http://surgicalneurologyint.com/surgicalint-articles/prediction-of-30%e2%80%91day-mortality-in-spontaneous-cerebellar-hemorrhage/
Abstract
Background:Cerebellar hemorrhage is a potentially life-threatening condition and an understanding of the factors influencing outcome is essential for sound clinical decision-making.
Methods:We retrospectively evaluated data from 50 consecutive patients who suffered a first spontaneous cerebellar hemorrhage (SCH) from 2005 to 2014, analysing their short-term outcomes and identifying possible clinical, radiological and therapeutic risk factors for poor prognosis and death within 30 days.
Results:Among 50 patients with first SCH, the mean age was 72 ± 10 years. Median Glasgow Coma Scale (GCS) score on admission was 11 [interquartile range (IQR) = 7–11]. Among 50 patients, 19 patients (38%) underwent surgical hemorrhage evacuation with placement of an external ventricular drain (EVD), 12 patients (24%) received an EVD only and 19 patients (38%) were treated conservatively. The 30-day mortality rate was 36%. In multivariate analysis only the GCS score on admission was a significant predictor of 30-day mortality [odds ratio (OR) = 0.598; 95% confidence interval (CI) = 0.406–0.879; P = 0.009]. For prediction of 30-day mortality, receiver operating characteristic curve analysis confirmed that the best cut-off point was a GCS score of 10 on admission [area under the curve: 0.882, 95% CI = 0.717–1, P
Conclusion:Lower GCS score on admission was associated with increased 30-day mortality and poorer short-term outcome in patients with SCH. For patients with a GCS score
Keywords: 30-day mortality, cerebellar hemorrhage, prognosis, risk factors
INTRODUCTION
Cerebellar hemorrhages constitute about 10% of all intracerebral hemorrhages and about 15% of cerebellar strokes.[
We performed a study to analyze the short-term course of patients suffering from SCH and evaluate different possible outcome predictors, including patient- and treatment-related factors (medical versus surgical therapy) in our analysis.
MATERIALS AND METHODS
We retrospectively analyzed data from 50 consecutive patients treated in our tertiary academic centre between January 2005 and December 2014, all diagnosed with a first episode of isolated SCH. The exclusion criteria were secondary cerebellar hemorrhage caused by trauma, tumours, cavernomas, arteriovenous malformations or aneurysms, hemorrhagic transformation of a cerebellar infarct, the presence of accompanying supratentorial or brainstem hemorrhage, incomplete medical records and patients with initial absence of brainstem reflexes on admission.
Study design
This is a single-centre retrospective study. To evaluate the short-term outcome and define possible risk factors for an unfavourable prognosis among patients with first SCH, we created a data file including the following parameters: age, sex, initial neurologic status, the presence of cardiovascular comorbidities, previous consumption of antiplatelet and/or anticoagulant drugs, radiographic features and type of treatment (medical or surgical). The major end-points in our study were death within 30 days, functional status at follow-up (a maximum of 30 days after ictus) and recurrent cerebellar hemorrhage or ischemic stroke.
Neurological outcome was measured using the modified Rankin scale (mRS).[
The initial imaging data, mainly head computed tomography (CT) scans (LightSpeed VCT, GE Medical Systems, Milwaukee, WI, USA), were reviewed to determine the location and dimension of each SCH, the presence of intraventricular hemorrhage (IVH), and radiological signs of hydrocephalus. The hematoma volume was estimated on the initial head CT scan using the ABC/2 method, in which A is the greatest diameter on the largest hemorrhage slice, B is the diameter perpendicular to A and C is the number of axial slices with bleeding multiplied by the slice thickness.[
Treatment protocol
All patients were treated according to the standard of care in our institution. Alert patients with hemorrhage of maximal diameter <3 cm and without radiological evidence of hydrocephalus were treated conservatively in a neurointensive care unit. Unconscious patients and patients with a decreased consciousness level and hemorrhage diameter >3 cm were treated surgically, by means of evacuation of the bleeding through a suboccipital craniectomy and placement of an external ventricular drain (EVD). In cases of hemorrhage <3 cm but with IVH resulting in clinical and/or radiological signs of hydrocephalus, an EVD alone was placed. However, the decision for surgery was individualized in each case, taking into account patient comorbidities. Comatose patients with absent brainstem reflexes on admission received conservative therapy and/or palliative care and were excluded from the study because of the high likelihood of very poor outcome.
Statistical analysis
First, univariate logistic regression was used to assess the strength of association between the collected variables and short-term outcome, using 30-day mortality as a dependent variable. Independent variables included age, gender, GCS score, arterial hypertension, use of anticoagulant or antiplatelet medications (use of antiplatelet drugs and anticoagulants were evaluated separately), maximal hemorrhage diameter and hemorrhage location, presence of IVH, hydrocephalus and the choice of therapy (conservative/medical versus surgical). Multivariate analysis was performed as a second step, using 30-day mortality as a dependent variable, and incorporating independent variables showing significance or a trend towards significance in the univariate analysis. A Hosmer-Lemeshow test was used to assess the goodness of fit of the multivariate model. Results are reported as odds ratios (OR) together with 95% confidence intervals (CI). For the GCS on admission that appeared significant in the multivariate correlations, the receiver operating characteristic (ROC) curve analysis was applied for identification of the clinically relevant cut-off value. The diagnostic accuracy was judged based on the value of the area under the curve (AUC). The significance level was set to 0.05. Analyses were performed using Statistical Package for the Social Sciences (SPSS) version 22 (SPSS Inc., Chicago, IL, USA).
RESULTS
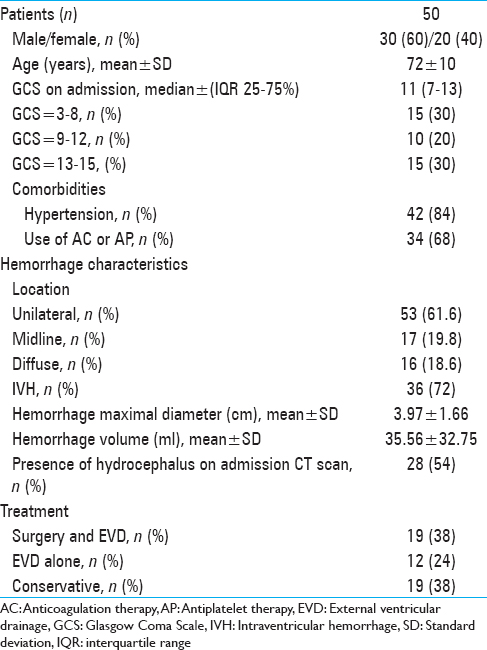
A total of 50 patients (mean age 72 ± 10 years; 30 males and 20 females) diagnosed with a first episode of SCH fulfilled the inclusion criteria. The median GCS score on admission was 11 [interquartile range (IQR) 7–13, range 3–15]. In this cohort, 84% of patients (n = 42) had a pre-existing diagnosis of arterial hypertension.
Use of a hemostasis-altering medication prior to ictus was identified in 34 patients (68%), who had been prescribed either antiplatelet (n = 20) or anticoagulant therapy (n = 14). 30 patients (61.6%) suffered a unilateral cerebellar hemorrhage and 12 patients (19.8%) experienced midline (vermian) hemorrhage. In 8 patients (18.6%) the hemorrhage was diffuse. The average hematoma diameter was 3.97 ± 1.66 cm, corresponding to a volume of 35.56 ± 32.75 ml (range 0.5–130.36 ml). IVH was found on the initial head CT scan of 36 patients (72%). Incipient hydrocephalus with compression of the fourth ventricle was apparent on the admission CT scan of 28 patients (54%). Among the 50 included patients, no patient developed a second hemorrhage or ischemic stroke within our short-term follow-up.
Nineteen patients (38%) underwent surgical evacuation of the cerebellar hemorrhage and placement of an EVD within 72 hours after ictus; 12 patients (24%) received an EVD alone and 19 (38%) were treated conservatively. The main characteristics of our cohort are summarized in
30-day outcome
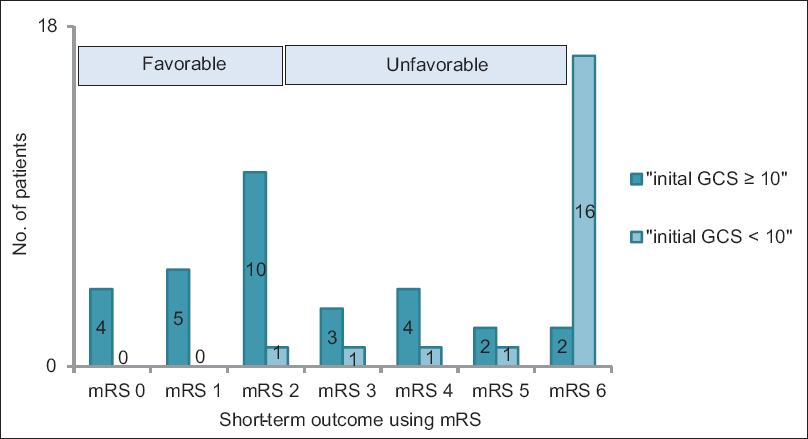
Eighteen patients died within 30 days of the occurrence of hemorrhage, representing a 30-day mortality of 36%. An unfavourable outcome (mRS ≥3) was documented in 30 patients (60%) at the end of short-term follow-up [
Logistic regression
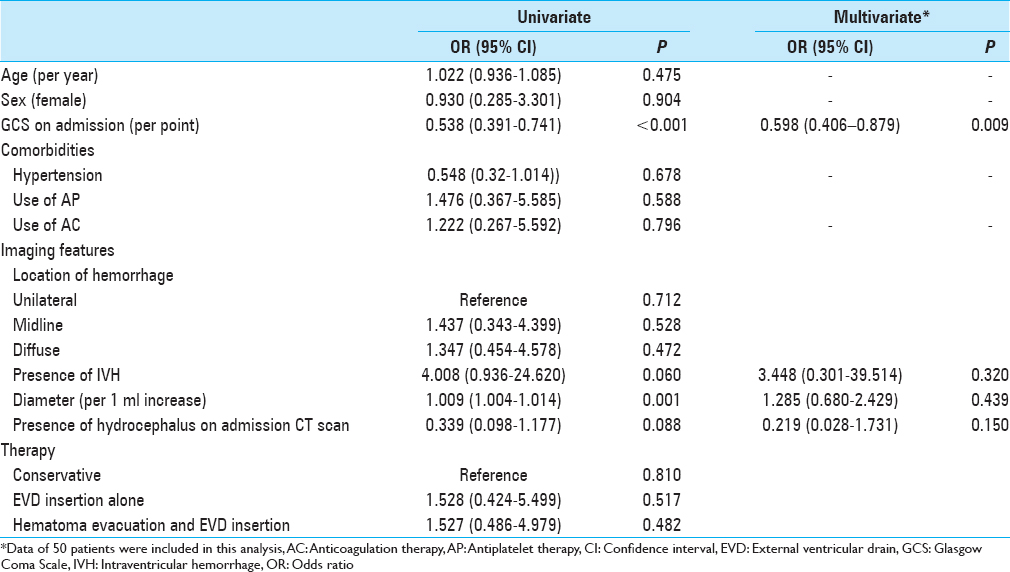
The univariate analysis showed admission neurological status according to GCS score and maximal diameter of the hemorrhage on initial head CT scan to be significant predictors of death within 30 days after ictus. The presence of hydrocephalus and IVH on initial head CT scan was not significantly associated with increased 30-day mortality [
The type of intervention, whether conservative or surgical, or with evacuation and EVD placement or with EVD placement alone, had no significant effect on outcome. However, it must be noted that the characteristics of the patients across each of the three treatment groups in this retrospective and non-randomized study were significantly different, particularly regarding the initial GCS score and the dimensions of the hemorrhage on initial head CT scan.
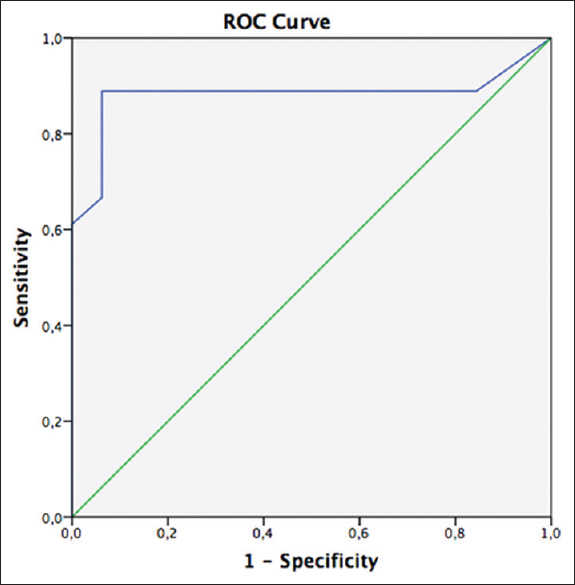
Performing a ROC analysis an optimal GCS score cut-off value of 10 was identified (AUC: 0.882, 95% CI = 0.717–1, P < 0.001) [
Figure 2
The ROC curve of the correlation between GCS on admission and 30-day mortality. The AUC of 0.882 points to the high diagnostic utility of GCS as a predictor of 30-day mortality. ROC curve analysis demonstrated a GCS score cut-off of 10 to be the best predictor of mortality at 30 days (AUC: 0.882, 95% CI = 0.717–1, P < 0.001)
A further analysis was performed to evaluate for the presence of a potential effect of the timing of surgical intervention on 30-day patient outcome. All 19 patients who underwent operative evacuation of the hemorrhage had this surgery within 72 hours of hemorrhage. This surgery was performed within 6 h and after 6 h of hemorrhage ictus in 14/19 patients (median 3.3 h) and 5/19 patients (median 34 h), respectively. There was no significant difference between these groups in terms of 30-day outcome (mortality 40% in patients operated within 6 h versus 43% in patients operated after 6 h, P = 0.912; morbidity 77% in patients operated within 6 h versus 80% in patients operated after 6 h, P = 0.947).
DISCUSSION
The 30-day mortality was 36% in our study, which is within the wide range of between 18 and 75% early morality after cerebellar hemorrhage reported in the literature.[
Arterial hypertension is strongly associated with the occurrence of cerebellar hemorrhage and it is known to pre-exist between 36 and 89% of cases.[
In relation to possible prognostic risk factors for early outcome, our work demonstrated that initial neurological status according to GCS score is the only significant predictors of 30-day mortality after SCH. The patients in our cohort with an initial GCS score <10 had an in-hospital mortality of about 89% and suffered an unfavourable outcome (mRS ≥3) in approximately 97% of cases.
The impaired conscious state seen in cerebellar hemorrhage can largely be attributed to the development of hydrocephalus, resulting in intracranial hypertension and/or direct brain stem compression.[
Hydrocephalus is a clinical emergency in patients with SCH and its early diagnosis and rapid treatment have a positive impact on outcome; the developing of hydrocephalus has also been described in many studies as a significant risk factor of unfavourable outcome.[
There is agreement that surgical evacuation of a cerebellar hemorrhage is indicated in unconscious patients or following deterioration of consciousness.[
We found no statistically significant correlation between advanced age and unfavourable outcome within the short-term follow-up period in this study. Some studies have found advanced age to be a strong predictor of an unfavourable outcome,[
Although not reflected in the results of our multivariate analysis, several studies have found particular radiological risk factors to be predictive of poor outcome, including large hemorrhage size,[
With regard to the timing of surgical intervention, there was no discernible, statistically significant effect on morbidity or mortality afforded by surgery within 6 h in comparison to surgery performed within 72 h.
The principal limitation of our study is its non-randomized and retrospective nature. We also cannot rule out unmeasured confounding from comorbidities and the fact that decision-making may not always have corresponded to standardized institutional protocols.
Several studies have investigated patient outcomes after spontaneous cerebellar and our findings align with a number of reports about predictors of short-term outcome following hemorrhage. Further, our study suggests that the strategy of evacuating hemorrhages >3 cm in diameter, with a low threshold for EVD placement in the context by clinically or radiologically diagnosed hydrocephalus, is a valid approach, which may help to reduce the size of hemorrhage and the degree of hydrocephalus and fourth ventricle compression. Addressing these factors leaves initial GCS score alone as a significant risk factor for poor outcome. In addition, we clearly show that those patients with an initial GCS score of <10 represent a particularly high-risk group.
CONCLUSION
The initial GCS score on admission is a significant predictor of short-term outcome and 30-day mortality in patients with SCH. For patients with a GCS score <10 on admission, it is important to weight the benefit of survival against the high risk of significant functional disability and poor quality of life that may be achieved by intensified or extended therapy. The findings in our study may help physicians to recognize patient-specific predictive factors for poor outcome and identify those patients who are likely to suffer early mortality after SCH regardless of medical intervention. Such early prognostication has significant consequences for medical decision-making, the counselling of family members and, by avoiding futile treatment, resource utilisation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
All authors certify that they have no affiliations with or involvement in any organisation or entity with any financial interest (such as honoraria; educational; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
1. Anderson CS, Chakera TM, Stewart-Wynne EG, Jamrozik KD. Spectrum of primary intracerebral haemorrhage in Perth, Western Australia, 1989-90: Incidence and outcome. J Neurol Neurosurg Psychiatry. 1994. 57: 936-40
2. Arnaout OM, Gross BA, Eddleman CS, Bendok BR, Getch CC, Batjer HH. Posterior fossa arteriovenous malformations. Neurosurg Focus. 2009. 26: E12-
3. Bahemuka M. Primary intracerebral haemorrhage and heart weight: A clinicopathologic case-control review of 218 patients. Stroke. 1987. 18: 531-6
4. Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D. Guidelines for the management of spontaneous intracerebral haemorrhage in adults: 2007 update: A guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007. 38: 2001-23
5. Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral haemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993. 24: 987-93
6. Brott T, Thalinger K, Hertzberg V. Hypertension as a risk factor for spontaneous intracerebral haemorrhage. Stroke. 1986. 17: 1078-83
7. Cohen ZR, Ram Z, Knoller N, Peles E, Hadani M. Management and outcome of non-traumatic cerebellar haemorrhage. Cerebrovasc Dis. 2002. 14: 207-13
8. Dammann P, Asgari S, Bassiouni H, Gasser T, Panagiotopoulos V, Gizewski ER. Spontaneous cerebellar haemorrhage—experience with 57 surgically treated patients and review of the literature. Neurosurg Rev. 2011. 34: 77-86
9. de Oliveira JG, Rassi-Neto A, Ferraz FA, Braga FM. Neurosurgical management of cerebellar cavernous malformations. Neurosurg Focus. 2006. 21: e11-
10. Dolderer S, Kallenberg K, Aschoff A, Schwab S, Schwarz S. Long-term outcome after spontaneous cerebellar haemorrhage. Eur Neurol. 2004. 52: 112-9
11. Donauer E, Loew F, Faubert C, Alesch F, Schaan M. Prognostic factors in the treatment of cerebellar haemorrhage. Acta Neurochir (Wien). 1994. 131: 59-66
12. Doukas A, Maslehaty H, Barth H, Hedderich J, Petridis AK, Mehdorn HM. A novel simple measure correlates to the outcome in 57 patients with intracerebellar hematomas. Results of a retrospective analysis. Surg Neurol Int. 2015. 6: 176-
13. Elkind MS, Mohr JP. Cerebellar haemorrhage. New Horiz. 1997. 5: 352-8
14. Firsching R, Huber M, Frowein RA. Cerebellar haemorrhage: Management and prognosis. Neurosurg Rev. 1991. 14: 191-4
15. Flaherty ML, Woo D, Haverbusch M, Sekar P, Khoury J, Sauerbeck L. Racial variations in location and risk of intracerebral haemorrhage. Stroke. 2005. 36: 934-7
16. Friedman JA, Piepgras DG, Duke DA, McClelland RL, Bechtle PS, Maher CO. Remote cerebellar haemorrhage after supratentorial surgery. Neurosurgery. 2001. 49: 1327-40
17. Han J, Lee HK, Cho TG, Moon JG, Kim CH. Management and outcome of spontaneous cerebellar haemorrhage. J Cerebrovasc Endovasc Neurosurg. 2015. 17: 185-93
18. Hill MD, Silver FL. Epidemiologic predictors of 30-day survival in cerebellar haemorrhage. J Stroke Cerebrovasc Dis. 2001. 10: 118-121
19. Kirollos RW, Tyagi AK, Ross SA, van Hille PT, Marks PV. Management of spontaneous cerebellar hematomas: A prospective treatment protocol. Neurosurgery. 2001. 49: 1378-
20. Kobayashi S, Sato A, Kageyama Y, Nakamura H, Watanabe Y, Yamaura A. Treatment of hypertensive cerebellar haemorrhage—surgical or conservative management?. Neurosurgery. 1994. 34: 246-
21. Konya D, Ozgen S, Pamir MN. Cerebellar haemorrhage after spinal surgery: Case report and review of the literature. Eur Spine J. 2006. 15: 95-9
22. Little JR, Tubman DE, Ethier R. Cerebellar haemorrhage in adults. Diagnosis by computerized tomography. J Neurosurg. 1978. 48: 575-9
23. Lui TN, Fairholm DJ, Shu TF, Chang CN, Lee ST, Chen HR. Surgical treatment of spontaneous cerebellar haemorrhage. Surg Neurol. 1985. 23: 555-8
24. McCormick WF, Rosenfield DB. Massive brain haemorrhage: A review of 144 cases and an examination of their causes. Stroke. 1973. 4: 946-54
25. Mezzadri JJ, Otero JM, Ottino CA. Management of 50 spontaneous cerebellar haemorrhages. Importance of obstructive hydrocephalus. Acta Neurochir (Wien). 1993. 122: 39-44
26. Park JS, Hwang JH, Park J, Hamm IS, Park YM. Remote cerebellar haemorrhage complicated after supratentorial surgery: Retrospective study with review of articles. J Korean Neurosurg Soc. 2009. 46: 136-43
27. Pollak L, Rabey JM, Gur R, Schiffer J. Indication to surgical management of cerebellar haemorrhage. Clin Neurol Neurosurg. 1998. 100: 99-103
28. Pong V, Chan KH, Chong BH, Lui WM, Leung GK, Tse HF. Long-term outcome and prognostic factors after spontaneous cerebellar haemorrhage. Cerebellum. 2012. 11: 939-45
29. Shenkin HA, Zavala M. Cerebellar strokes: Mortality, surgical indications, and results of ventricular drainage. Lancet. 1982. 2: 429-32
30. St Louis EK, Wijdicks EF, Li H. Predicting neurologic deterioration in patients with cerebellar hematomas. Neurology. 1998. 51: 1364-9
31. St Louis EK, Wijdicks EF, Li H, Atkinson JD. Predictors of poor outcome in patients with a spontaneous cerebellar hematoma. Can J Neurol Sci. 2000. 27: 32-6
32. Taneda M, Hayakawa T, Mogami H. Primary cerebellar haemorrhage. Quadrigeminal cistern obliteration on CT scans as a predictor of outcome. J Neurosurg. 1987. 67: 545-52
33. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974. 2: 81-4
34. Tsitsopoulos PP, Tobieson L, Enblad P, Marklund N. Prognostic factors and long-term outcome following surgical treatment of 76 patients with spontaneous cerebellar haematoma. Acta Neurochir. 2012. 154: 1189-95
35. van der Hoop RG, Vermeulen M, van Gijn J. Cerebellar haemorrhage: Diagnosis and treatment. Surg Neurol. 1988. 29: 6-10
36. van Loon J, Van Calenbergh F, Goffin J, Plets C. Controversies in the management of spontaneous cerebellar haemorrhage. A consecutive series of 49 cases and review of the literature. Acta Neurochir. 1993. 122: 187-93
37. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988. 19: 604-7
38. Wijdicks EF, St Louis EK, Atkinson JD, Li H. Clinician's biases toward surgery in cerebellar hematomas: An analysis of decision-making in 94 patients. Cerebrovasc Dis. 2000. 10: 93-6
39. Zieger A, Vonofakos D, Steudel WI, Dusterbehn G. Nontraumatic intracerebellar hematomas: Prognostic value of volumetric evaluation by computed tomography. Surg Neurol. 1984. 22: 491-4






Bea Smith
Posted December 10, 2017, 7:12 am
More of studies like this should be done.