- Department of Neurological Surgery, University of California, Davis [Retired], Sacramento, California.
DOI:10.25259/SNI_449_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Goldsmith HS. Prevention of thromboembolism: A personal journey. Surg Neurol Int 27-Sep-2019;10:185
How to cite this URL: Goldsmith HS. Prevention of thromboembolism: A personal journey. Surg Neurol Int 27-Sep-2019;10:185. Available from: https://surgicalneurologyint.com/surgicalint-articles/9677/
Over the past several decades, deep vein thrombosis (DVT) and pulmonary emboli (PEs) have been treated with anticoagulants, to (1) decrease the coagulability of blood and (2) to compress the veins in the lower extremities to intermittently direct superficial blood flow into the deeper and larger veins in the legs. Neither of these methods seem to have proven very effective. A new clinical approach appears justified.
My interest in DVT and PEs began when I was a 1st year surgical resident at the Boston City Hospital where I had the opportunity to perform my first cholecystectomy. The patient did well following surgery and we developed a warm friendship. On her 6th postoperative day [patients were routinely hospitalized for a week following surgery at that time], I told her she would be released from the hospital the following day. I left her bedside and went to the nurse’s station and while writing notes were suddenly informed by a student nurse that my gallbladder patient had gotten out of bed and collapsed. I raced to the patient and found her on the floor at the foot of her bed. She failed to respond to my efforts to revive her. I placed her back in her bed and because she had no previous history of heart disease, I believed she had suffered a fatal pulmonary embolism, which was later confirmed at autopsy.
After placing her in bed, I removed the compressive ACE bandages from both her legs. Compressive bandages were routinely applied at the hospital to prevent DVT. Unfortunately, the hospital’s elastic bandages, weakened by repeated washings and reused, had lost their elasticity. As I took the bandages off the patient’s legs, tourniquet marks were observed on both legs. The inelasticity of the bandage material had obviously allowed the venous flow to slow within both legs and had resulted in a DVT and PE.
Losing this patient was devastating, and it stimulated my determination to develop a technique which could minimize the development of DVT and PEs.[
Based on a search of literature, it appeared that the only means for increasing venous velocity in the legs were either by a mechanical or physiological method. The physiological method to increase venous velocity in the legs was first described by Walters in 1930 at the Mayo Clinic.[
In an attempt to increase venous velocity by mechanical methods, H.P. Wright in 1952 administered radioactive salt (Na24Cl) intravenously into the foot of patients under standard conditions of temperature, rest, and position. By placing a radiation counter on a patient’s groin, Wright was able to accurately determine the time it took for the venous blood flow from the point of injection in the foot to its passage under the radiation counter in the groin. He showed that there was 100% increase in the speed of venous blood flow shortly after carrying out plantar and dorsi flexion of the feet. This increase in venous speed occurred within 1 min following the flexion of the feet.[
it is of interest that such a profound result can be produced by so small a movement as dorsi and plantar flexion of the foot, and it appears that this simple exercise offers a satisfactory method of insuring a rapid venous flow rate in those patients who must be kept recumbent. Even the old and frail or seriously ill can be required several times a day to perform the simple pedaling movement shown to have been so effective in combatting venous stasis.
Wright’s statement clearly showed that a device had to be developed to increase venous velocity in the lower extremities. Our laboratory performed the same experiment to confirm Wright’s observation, and it was also found that there was 100% increase in the venous speed after only 1 min of dorsi and plantar flexion.
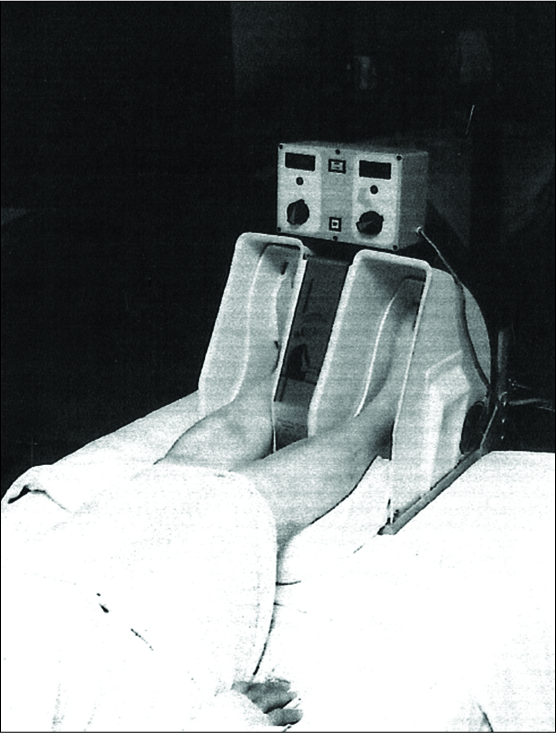
Based on Wright’s observation, our laboratory over a 16-year period evaluated 17 models for increasing venous velocity. The model finally selected was named the Pedi-Pulsor [
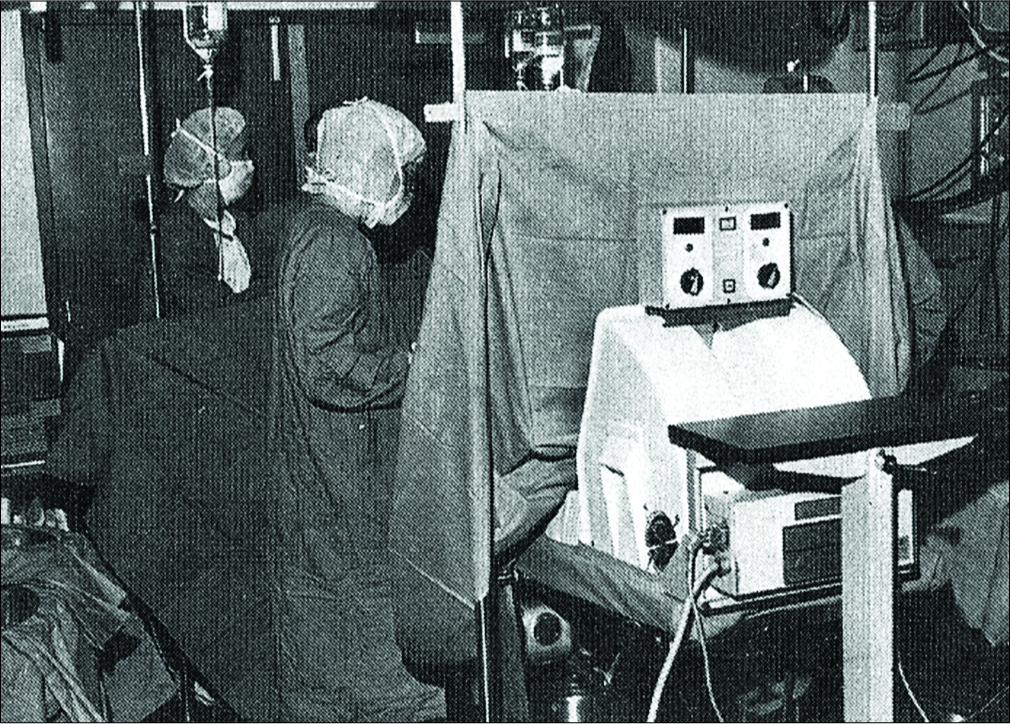
The Pedi-Pulsor was initially used by several surgical patients and was subsequently tested in London. The British had discovered that DVT could develop in patients during operative procedures and they began to use the Pedi- Pulsor to determine if plantar and dorsi flexion during an operation could lessen the incidence of DVT during an operative procedure. Sixty-six patients were involved in their study. Patients were randomly divided into a control and Pedi-Pulsor group and were treated in the same manner preoperatively and postoperatively. The only difference between the Pedi-Pulsor and control groups was that during a surgical procedure, the control group had their legs immobile on the operating table, whereas patients in the Pedi-Pulsor group had their feet placed in the device which allowed plantar and dorsi flexion during the course of the operation [
The Pedi-Pulsor’s clinical success in the British study led the American Hospital Company to consider the commercialization of the device. However, the company was also considering at that time the commercialization of an intermittent compressive long leg garment. The company eventually chose the leg garment over the Pedi- Pulsor. No other companies showed interest in the Pedi- Pulsor and, unfortunately, the company that built the device went out of business. There was nothing further I could do at this point to promote the Pedi-Pulsor and its potential to prevent DVT and pulmonary emboli. My involvement with the prevention of thromboembolism had come to an end.
Over the years, my focus turned to other clinical and research questions until 2008 when the United States Department of Health and Human Services sent a 41-page brochure to doctors throughout the United States.[
In response to the government’s “call to action in 2008,” I contacted authorities in Washingon and told them that I believed the only way to prevent DVT and PEs was by increasing the venous velocity in the lower extremities. I explained that anticoagulation would decrease the solubility of blood, but it would not increase velocity of the blood stream. I also warned that intermittent compression of the legs over an extended period might induce irritation in the section of a vein wall, increasing the possibility of the development of a blood clot. I explained this possibility by claiming that the blood flow within a vein flows at different speeds with the fastest rate of flow occurring in the center of the vein and the slowest blood flow rate flowing adjacent to the inside of the venous wall. The slow blood flow might increase the possibility that platelets could adhere at this location, causing a blood clot to develop at this site that could result in a DVT and subsequent PE.
Although the governmental authorities seemed receptive to my observations and suggestions, I was told that since President Obama had just been inaugurated (2008), the health agency would not be able to address any proposals until they were certain of their future funding.
I assumed that the strong request by officials in the United States Department of Health and Human Services would stimulate clinicians and researchers involved with problems of thromboembolism to respond to this urgent governmental initiative. However, 11 years (2008–2019) have now passed since the government’s “call to action” and apparently, there has been no significant improvement in the prevention of DVT and PEs.
The New England Journal of Medicine recently published a study of 2000 patients who were treated with a combination of anticoagulation plus leg compression in the hope that this combination of treatment would improve the present-day results of thromboembolic prevention. The study unfortunately concluded that the combination of anticoagulation plus leg compression “did not significantly lower the incidence of lower limb deep vein thrombosis”.[
During the past 11 years (2008–2019), following the government’s call to action, nothing of clinical significance pertaining to the lowering of the incidence of thromboembolism has apparently occurred. It seems reasonable to suggest that the government could and should now take an active role in an attempt to lessen the incidence of thromboembolism. The government has the resources to develop an instrument equal, or even superior, to the Pedi- Pulsor that could be evaluated at the National Health Institute (NIH) Hospital in Washington. Clinical evaluation at the NIH clinical institution would not require a grant application (an activity which slows down all projects). If the government develops a device that was found to be effective in lowering the incidence of DVTs and pulmonary emboli, it could be marketed nationally, even internationally (comparable to the US government selling military equipment to countries throughout the world). The government might find the project to be lucrative.
If we are to lower the incidence of DVT and PEs, it is hoped that the government will become involved. The government’s participation could lead to a major therapeutic advance. We will await to see if this suggestion is evaluated by governmental authorities in Washington. There is every reason to believe that such an important government project could be accomplished.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management.
References
1. Arabi YM, Al-Hameed F, Burns KEA, Mehta S, Alsolamy SJ, Alshahrani MS. Adjunctive intermittent pneumatic compression for venous thromboprophylaxis. N Engl J Med. 2019. 380: 1305-15
2. Goldsmith HS. The prophylaxis of thromboembolism. Surg Gynecol Obstet. 1966. 122: 799-804
3. Goldsmith HS. Thromboembolic disease-emphasis on prevention. Med Times. 1968. 96: 774-84
4. Scurr JH, Robbe IJ, Ellis H, Goldsmith HS. Simple mechanical method for decreasing the incidence of thromboembolism. Am J Surg. 1981. 141: 582-5
5. .editors. US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville: US Department of Health and Human Services; 2008. p.
6. Walters W. A method of reducing the incidence of fatal postoperative pulmonary embolism. Surg Gynecol Obstet. 1930. 50: 154-9
7. Wright HP, Osborn SB. Effect of posture on venous velocity, measured with 24NaCl. Br Heart J. 1952. 14: 325-30