- Department of Neurosurgery, Henry Ford Hospital, Detroit, Michigan, USA
- Department of Public Health Sciences, Henry Ford Hospital, Detroit, Michigan, USA
Correspondence Address:
Azam Basheer
Department of Neurosurgery, Henry Ford Hospital, Detroit, Michigan, USA
DOI:10.4103/sni.sni_5_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Azam Basheer, Mohammed Alsaidi, Lonni Schultz, Mokbel Chedid, Muwaffak Abdulhak, Donald Seyfried. Preventive effect of tamsulosin on postoperative urinary retention in neurosurgical patients. 10-May-2017;8:75
How to cite this URL: Azam Basheer, Mohammed Alsaidi, Lonni Schultz, Mokbel Chedid, Muwaffak Abdulhak, Donald Seyfried. Preventive effect of tamsulosin on postoperative urinary retention in neurosurgical patients. 10-May-2017;8:75. Available from: http://surgicalneurologyint.com/surgicalint-articles/preventive-effect-of-tamsulosin-on-postoperative-urinary-retention-in-neurosurgical-patients/
Abstract
Background:Postoperative urinary retention (POUR) is common in neurosurgical patients. The use of alpha-blockade therapy, such as tamsulosin, has benefited many patients with a history of obstructive uropathy by decreasing lower urinary tract symptoms such as distension, infections, and stricture formation, as well as the incidence of POUR. For this study, we targeted patients who had undergone spinal surgery to examine the prophylactic effects of tamsulosin. Increased understanding of this therapy will assist in minimizing the morbidity of spinal surgery.
Methods:We enrolled 95 male patients undergoing spine surgery in a double-blind, randomized, placebo-controlled trial. Patients were randomly assigned to receive either preoperative tamsulosin (N = 49) or a placebo (N = 46) and then followed-up prospectively for the development of POUR after removal of an indwelling urinary catheter (IUC). They were also followed-up for the incidence of IUC reinsertions.
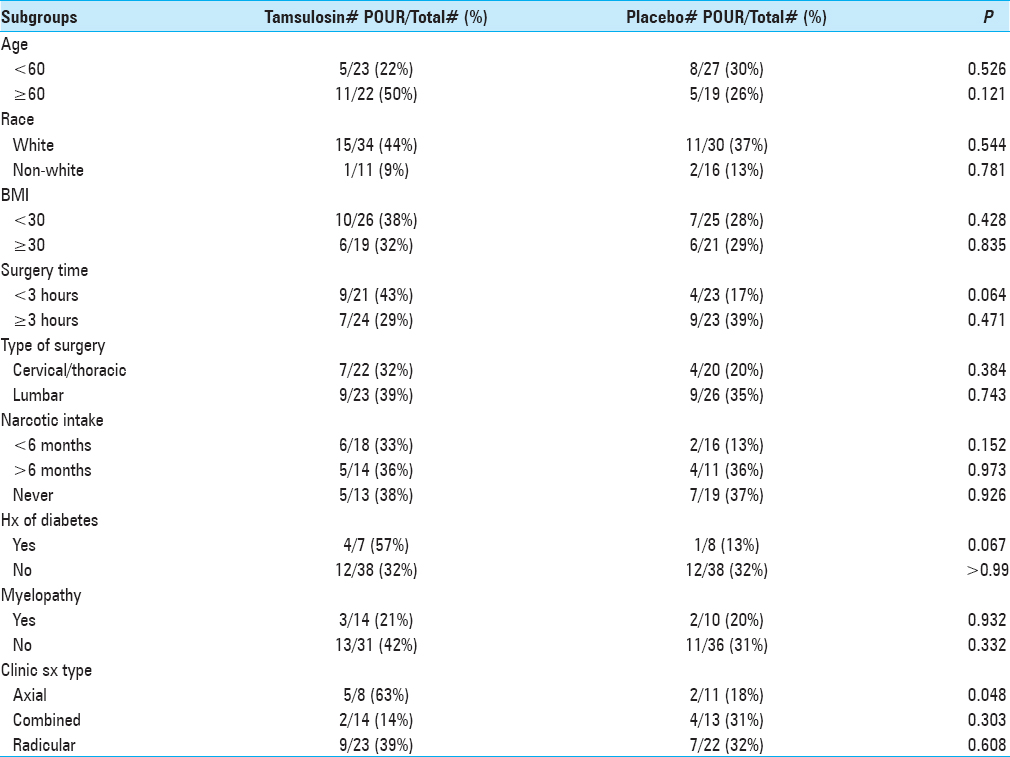
Results:The rate of developing POUR was similar in both the groups. Of the 49 patients given tamsulosin, 16 (36%) developed POUR compared to 13 (28%) from the control group (P = 0.455). In the control group, 5 (11%) patients had IUC re-inserted postoperatively, whereas 7 (14%) patients in the tamsulosin group had IUC re-inserted postoperatively (P = 0.616). In patients suffering from axial-type symptoms (i.e., mechanical back pain), 63% who received tamsulosin and 18% from the control group (P = 0.048) developed POUR.
Conclusion:Overall, there was no statistically significant difference in the rates of developing POUR among patients in either group. POUR is caused by a variety of factors, and further studies are needed to shed light on its etiology.
Keywords: Adrenergic alpha-antagonists, indwelling urinary catheter, neurosurgery, postoperative urinary retention, tamsulosin, urinary retention
INTRODUCTION
Postoperative urinary retention (POUR) has been defined as the inability to void despite a full bladder.[
Advances in pharmacology, specifically the institution of selective alpha-blockers (e.g., tamsulosin), have provided feasible, noninvasive interventions in the treatment of benign prostatic hyperplasia (BPH).[
The purpose of our study was to determine if pharmacological intervention using tamsulosin, administered perioperatively, would reduce the incidence of POUR in men undergoing elective spinal surgery.
MATERIALS AND METHODS
Study design and participants
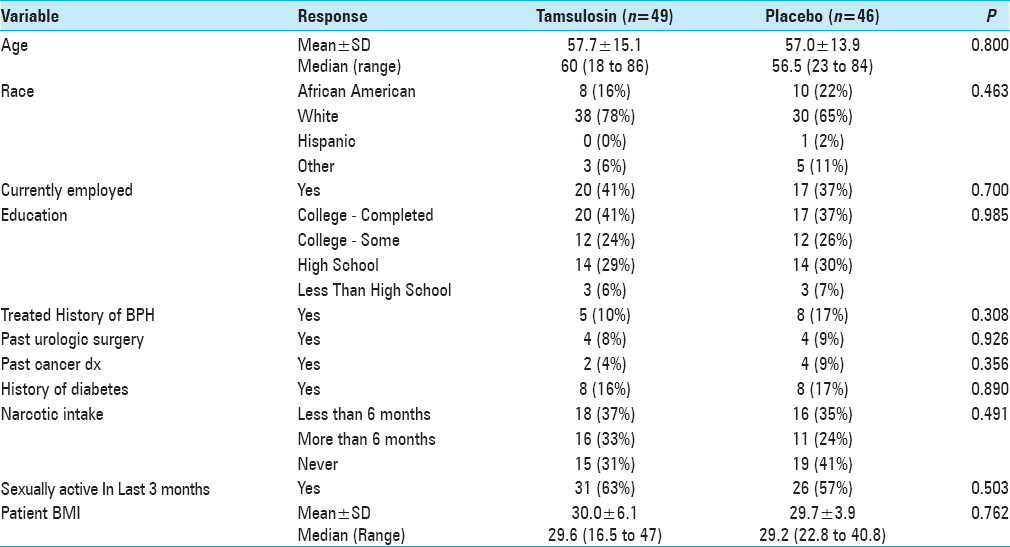
This was a double-blind, randomized, placebo-controlled trial carried out from April 2012 to January 2013. Ninety-five male neurosurgical patients undergoing spine surgery in our hospital were randomly assigned to receive preoperative tamsulosin (Flomax®) and then followed up prospectively for the development of POUR. This study was approved by the Henry Ford Hospital Institutional Review Board (IRB # 6893).
The study was introduced to eligible male patients between the ages of 18 and 80 who presented to the Neurosurgery Department Clinic of Henry Ford Hospital for elective spinal surgery. Patients were excluded from the study if they met any of the criteria in
Randomization, masking, and data collection
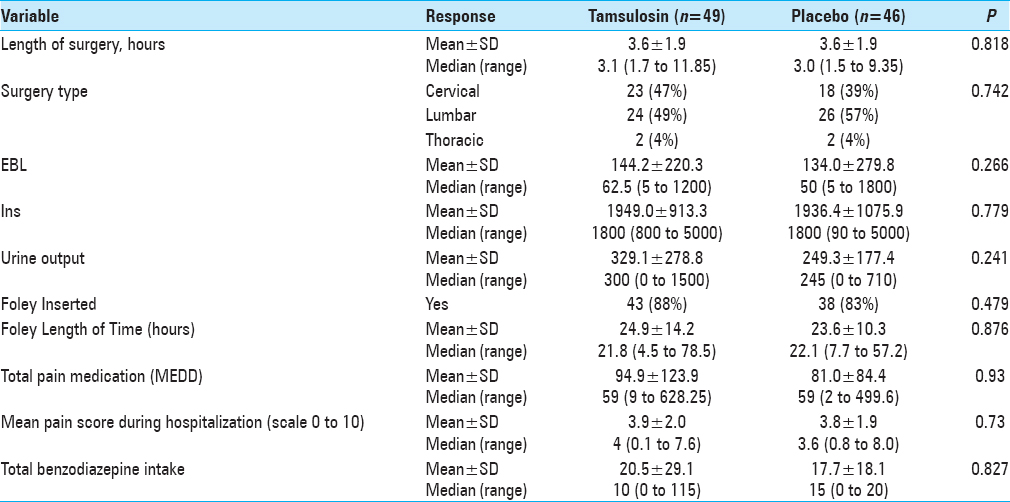
Patients who enrolled were then randomly assigned to either tamsulosin or placebo pills using a computer-generated randomization list, which was stratified by age (<50, 50–64, and 65+ years). Patients and study assessors were masked to the treatment allocation. Medication (0.4 mg of tamsulosin or placebo) was administered orally 48 h before the surgery and the night before surgery. On the day of the surgery, patients were monitored by the project team while in hospital as well as upon discharge. The amount of postoperative urinary volume was monitored using the standard bladder ultrasound until post-void residual of < 250 ml was reached. Patients were continued on medication/placebo every night while inpatient until the Foley placed during surgery was removed, typically on postoperative day 1. Medication/placebo was discontinued if no Foley was placed during surgery. No patients were sent home on the medication/placebo postoperatively. In all cases, intravenous fluids were administered in the operating room before the anesthetic was given and continued postoperatively until the day after surgery (12–18 h).
Patients were placed on a pain-control pump (PCA) of either morphine or Dilaudid postoperatively and subsequently weaned to oral narcotics on postoperative day 1. Patients’ charts were reviewed and total narcotics dosage and benzodiazepine doses were calculated. All patients were followed during their postoperative stay for any voiding difficulties, and urinary retention was recorded.
Definition of postoperative urinary retention
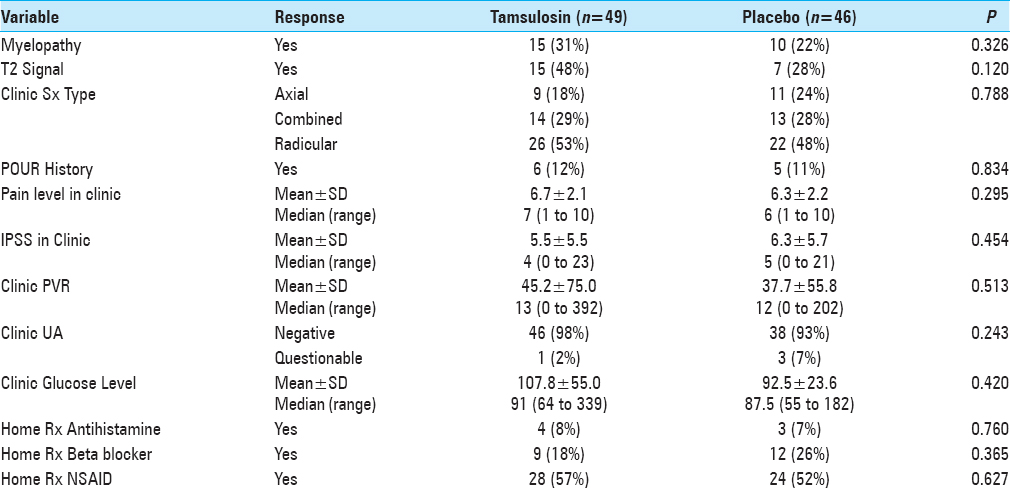
POUR, as per the hospital protocol, was defined as an initial post-void residual (PVR) greater than 250 ml using bladder ultrasonography (BVI 3000, Verathon) 6 h after the removal of IUCs inserted during surgery. Straight catheterization was performed for patients with PVR greater than 250 ml every 6 h. For patients with the third PVR greater than 250 ml, IUCs were reinserted. Patients were then discharged and instructed to return to the urology clinic in 5–7 days for follow-up. Subsequently, patients’ records were reviewed for multiple variables [Tables
RESULTS
The incidence of POUR in all patients was 32% (i.e., based on our definition of first PVR greater than 250 ml). The rate of developing POUR was similar in the placebo and treatment groups, with 16 patients (36%) in the tamsulosin group developing POUR compared to 13 patients (28%) from the placebo group (P = 0.455). The rates of Foley reinsertion were also comparable for the two treatment groups (14% for tamsulosin vs 11% for placebo, P = 0.616). No differences were observed between the two groups for length of stay (LOS) (P = 0.755) and discharge disposition (P = 0.394). For those with predominantly axial back pain, using tamsulosin postoperatively resulted in POUR in 63% versus 18% of patients receiving placebo (P = 0.048).
DISCUSSION
POUR is a well-established and commonly encountered problem across all surgical specialties (frequency of 5% to 75%), but has not been studied extensively in spinal neurosurgical patients.[
Tamsulosin was first developed in Japan and marketed in 1996 under the trade name Flomax®.[
We are aware of only four randomized trials that assessed the effectiveness of tamsulosin administered perioperatively to prevent POUR, however, none involved spine surgery.[
Madani et al. followed 232 male patients aged 18 to 50 years of age undergoing varicocelectomy, inguinal herniorrhaphy, and scrotal surgery.[
The etiology of urinary retention following spine surgery is likely neurological manipulation.[
We previously found a trend of increased retention following cervicothoracic surgeries compared with lumbar surgeries, which may be due to damaged spinal cord fibers, however, the type of surgery had little to no bearing on the development of POUR in the tamsulosin and placebo groups.[
Finally, it is widely believed that the use of pain medications contributes to the development of POUR.[
CONCLUSION
Despite largely negative study results, tamsulosin has shown promise when used in other specialties as well as positive trends in certain patient subgroups. Further and larger clinical trials are needed to investigate the effectiveness of such medications in patients undergoing spinal surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ahmad MM, Wani HA, Jeelani A, Thakur S, Waseem M, Nazir I. Preventive effect of tamsulosin on postoperative urinary retention in benign anorectal surgeries. Saudi Surg J. 2014. 2: 33-7
2. Alsaidi M, Guanio J, Basheer A, Schultz L, Adulhak M, Nerenz D. The incidence and risk factors for postoperative urinary retention in neurosurgical patients. Surg Neurol Int. 2013. 4: 61-
3. Balderi T, Mistraletti G, D’Angelo E, Carli F. Incidence of postoperative urinary retention (POUR) after joint arthroplasty and management using ultrasound-guided bladder catheterization. Minerva Anestesiol. 2011. 77: 1050-7
4. Baldini G, Bagry H, Aprikian A, Carli F. Postoperative urinary retention anesthetic and perioperative considerations. Anesthesiology. 2009. 110: 1139-57
5. Boulis NM, Mian FS, Rodriguez D, Cho E, Hoff JT. Urinary retention following routine neurosurgical spine procedures. Surg Neurol. 2001. 55: 23-7
6. Darrah DM, Griebling TL, Silverstein JH. Postoperative urinary retention. Anesthesiol Clin. 2009. 27: 465-84
7. Getliffe K. Care of urinary catheters. Nurs Stand. 1996. 11: 47-50
8. Haley RW, Hooton TM, Culver DH, Stanley RC, Emori TG, Hardison CD. Nosocomial infections in U.S. hospitals, 1975-1976; Estimated frequency by selected characteristics of patients. Am J Med. 1981. 70: 947-59
9. Jang JH, Kang SB, Lee SM, Park JS, Kim DW, Ahn S. Randomized controlled trial of tamsulosin for prevention of acute voiding difficulty after rectal cancer surgery. World J Surg. 2012. 36: 2730-7
10. Jellish WS, Thalji Z, Stevenson K, Shea J. A prospective randomized study comparing short- and intermediate-term perioperative outcome variables after spinal or general anesthesia for lumbar disk and laminectomy surgery. Anesth Analg. 1996. 83: 559-64
11. Kebapci N, Yenilmez A, Efe B, Entok E, Demirustu C. Bladder dysfunction in type 2 diabetic patients. Neurourol Urodyn. 2007. 26: 814-9
12. Keita H, Diouf E, Tubach F, Brouwer T, Dahmani S, Mantz J. Predictive factors of early postoperative urinary retention in the postanesthesia care unit. Anesth Analg. 2005. 101: 592-6
13. Kneist W, Junginger T. Long-term urinary dysfunction after mesorectal excision: A prospective study with intraoperative electrophysiological confirmation of nerve preservation. Eur J Surg Oncol. 2007. 33: 1068-74
14. Lee KS, Lim KH, Kim SJ, Choi HJ, Noh DH, Lee HW. Predictors of successful trial without catheter for postoperative urinary retention following non-urological surgery. Int Neurourol J. 2011. 15: 158-65
15. Liang CC, Lee CL, Chang TC, Chang YL, Wang CJ, Soong YK. Postoperative urinary outcomes in catheterized and non-catheterized patients undergoing laparoscopic-assisted vaginal hysterectomy – A randomized controlled trial. Int Urogynecol J. 2009. 20: 295-300
16. Madani A, Aval H, Mokhtari G, Nasseh H, Esmaeili S, Shakiba M. Effectiveness of tamsulosin in prevention of post-operative urinary retention: A randomized double-blind placebo-controlled study. Int Braz J Urol. 2014. 40: 30-6
17. McLain RF, Kalfas I, Bell GR, Tetzlaff JE, Yoon HJ, Rana M. Comparison of spinal and general anesthesia in lumbar laminectomy surgery: A case-controlled analysis of 400 patients. J Neurosurg Spine. 2005. 2: 17-22
18. Mohammadi-Fallah M, Hamedanchi S, Tayyebi-Azar A. Preventive effect of tamsulosin on postoperative urinary retention. Korean J Urol. 2012. 53: 419-23
19. Olsen S, Nielsen J. A study into postoperative urine retention in the recovery ward. Br J Anaesth Recov Nurs. 2007. 8: 91-5
20. Petros JG, Rimm EB, Robillard RJ, Argy O. Factors influencing postoperative urinary retention in patients undergoing elective inguinal herniorrhaphy. Am J Surg. 1991. 161: 431-3
21. Petros JG, Rimm EB, Robillard R. Factors influencing urinary tract retention after elective open cholecystectomy. Surg Gynecol Obstet. 1992. 174: 497-500
22. Platt R, Polk BF, Murdock B, Rosner B. Mortality associated with nosocomial urinary-tract infection. N Engl J Med. 1982. 307: 637-42
23. Roehrborn CG, Siami P, Barkin J, Damiao R, Major-Walker K, Morrill B. The effects of dutasteride, tamsulosin and combination therapy on lower urinary tract symptoms in men with benign prostatic hyperplasia and prostatic enlargement: 2-year results from the CombAT study. J Urol. 2008. 179: 616-21
24. Schaeffer AJ. Catheter-associated bacteriuria. Urol Clin North Am. 1986. 13: 735-47
25. Siroky M. The aging bladder. Rev Urol. 2004. 6: S3-7
26. Smith NK, Albazzaz MK. A prospective study of urinary retention and risk of death after proximal femoral fracture. Age Ageing. 1996. 25: 150-4
27. Sullivan NM, Sutter VL, Mims MM, Marsh VH, Finegold SM. Clinical aspects of bacteremia after manipulation of the genitourinary tract. J Infect Dis. 1973. 127: 49-55
28. Tehranchi A, Rezaei Y, Shojaee R. Tolterodine to relieve urinary symptoms following transurethral resection of the prostate: A double-blind placebo-controlled randomized clinical trial. Korean J Urol. 2014. 55: 260-4
29. Toyonaga T, Matsushima M, Sogawa N, Jiang SF, Matsumura N, Shimojima Y. Postoperative urinary retention after surgery for benign anorectal disease: Potential risk factors and strategy for prevention. Int J Colorectal Dis. 2006. 21: 676-82
30. Warren JW, Platt R, Thomas RJ. Antibiotic irrigation and catheter-associated urinary-tract infections. N Engl J Med. 1978. 299: 570-3
31. Williams MP, Wallhagen M, Dowling G. Urinary retention in hospitalized elderly women. J Gerontol Nurs. 1993. 19: 7-14