- Faculty of Medicine, University of Jordan, Jordan
- Department of Neurosurgery, University of Jordan, Jordan
- Department of Neurosurgery, Pontifical Catholic University of Campinas (PUC-CAMPINAS), São Paulo, Brazil
Correspondence Address:
Tareq M. A. Kanaan
Faculty of Medicine, University of Jordan, Jordan
Department of Neurosurgery, University of Jordan, Jordan
DOI:10.4103/2152-7806.194512
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ahmed M. A. Altibi, Raed A. H. Qarajeh, Telmo A. B. Belsuzarri, Walid Maani, Tareq M. A. Kanaan. Primary cerebral echinoccocosis in a child: Case report – Surgical technique, technical pitfalls, and video atlas. 21-Nov-2016;7:
How to cite this URL: Ahmed M. A. Altibi, Raed A. H. Qarajeh, Telmo A. B. Belsuzarri, Walid Maani, Tareq M. A. Kanaan. Primary cerebral echinoccocosis in a child: Case report – Surgical technique, technical pitfalls, and video atlas. 21-Nov-2016;7:. Available from: http://surgicalneurologyint.com/?post_type=surgicalint_articles&p=8176
Abstract
Background:Hydatid disease is a life-threatening parasitic infestation caused by Echinococcus granulosus. Infection with E. granulosus typically results in the formation of hydatid cysts in the liver, lungs, kidney, and spleen. Primary intracranial hydatid cyst disease is extremely rare. Here, we are reporting an unusual case of Echinococcus, where the only identifiable lesion was a hydatid cyst in the brain without liver or lung involvement. We are also providing a description for the surgical technique used to remove the cyst, highlighting the possible surgical pitfalls.
Case Description:The patient is a 13-year-old male with a history of progressive headache for 1 month. Intracranial hydatid cyst was suspected based on computed tomography and magnetic resonance imaging findings. The cyst was delivered without rupture using hydrostatic dissection (Dowling's technique), and pathological analysis confirmed the diagnosis. Postoperatively, the patient showed marked neurological improvement and all signs and symptoms resolved.
Conclusion:Intracranial hydatid cyst is very rare. Nevertheless, it should always be considered as a differential diagnosis in cerebral cystic lesions, especially in children. The surgical technique used to remove the cyst appears to be safe. However, several precautions must be applied intraoperatively to avoid the catastrophe of cyst rupture.
Keywords: Cerebral hydatid cyst, children, Dowling–Orlando technique, Echinococcus
INTRODUCTION
Human hydatid disease is caused by the larval form of Echinococcus granulosus. Dogs are definitive hosts of E. granulosus whereas sheep are typically the intermediate hosts.[
After ingestion of contaminated food, the embryos migrate through the portal system to the liver, and later to the lungs.[
Brain involvement is unusual, seen in only 2–3% of systematic hydatid disease, and forms 2% of all intracranial mass lesions.[
CASE PRESENTATION
A 13-year-old male, previously healthy, right-handed patient, presented to the outpatient department with a 1-month history of a progressive headache of mild-to-moderate intensity. Headache was initially right-sided and then progressed to become bilateral. Headache was associated with nausea, blurring of vision, and photophobia.
Neurological exam revealed ataxic gait, left-sided pronator drift, left-sided dysdiadochokinesia, and hypoesthesia in the distribution of the left mandibular nerve. Fundoscopy showed mild bilateral optic disk swelling. Examination of the visual field revealed the presence of multiple points of absolute defects in the visual field involving peripheral temporal area in a ring-like distribution, and various points of absolute scotomas in the nasal field.
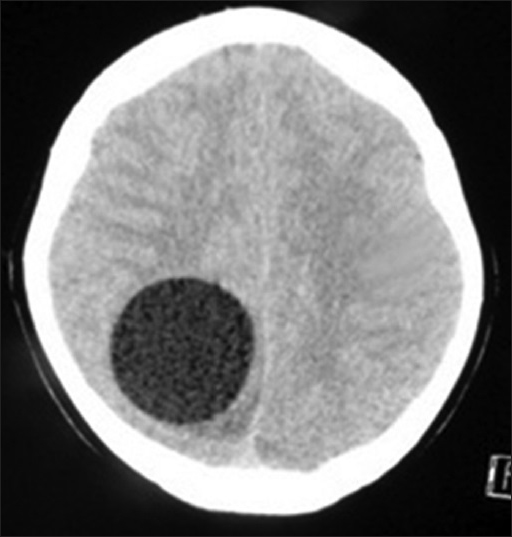
Axial brain computed tomography (CT) [
Figure 1
Axial brain CT without contrast showing a well-defined, CSF dense, solitary, cystic lesion located in the right parieto-occipital region. The lesion is causing mild mass effect on the midline which appears slightly shifted to the left. Cortical sulci are effaced in the right hemisphere and minor compression of the posterior body of the right lateral ventricle is evident. No focal calcifications, septations, or daughter cysts are evident in the lesion
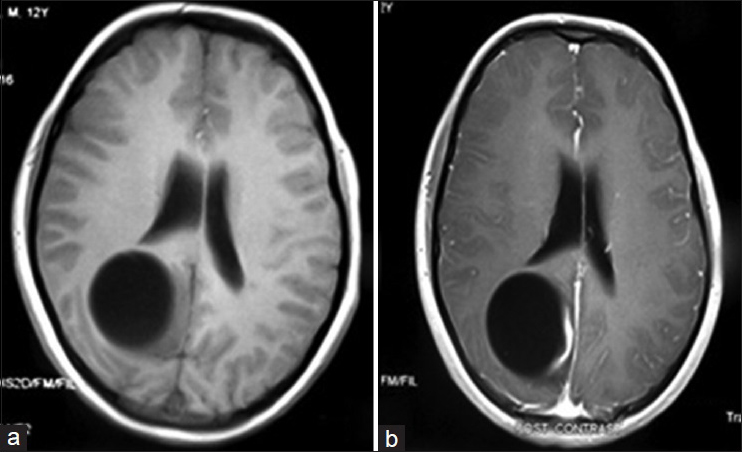
Figure 2
(a) T1W brain MRI without contrast shows an oval-shaped, intra-axial, cystic lesion at the right parietoocipital region. Mild hypointensity within the adjacent brain parenchyma can be detected in the parasagittal occipital lobe, which suggests the presence of vasogenic edema or inflammatory process. There is no apparent soft tissue component. (b) T1W brain MRI with contrast shows a linear, mural enhancement on the medial aspect of the cysts parasagittal border suggesting an inflammatory process
Thorax and abdominal CT, chest X-ray, and magnetic resonance imaging (MRI) of the spine were performed to find a primary focus, however, the results were negative. On complete blood count, no eosinophilia was detected (eosinophils count = 0.45%), and neutrophilia was the only abnormal finding (neutrophils count = 68%). Furthermore, liver function tests and electrolyte panel were within normal limits. IgG anti-Echinococcus antibodies were also negative.
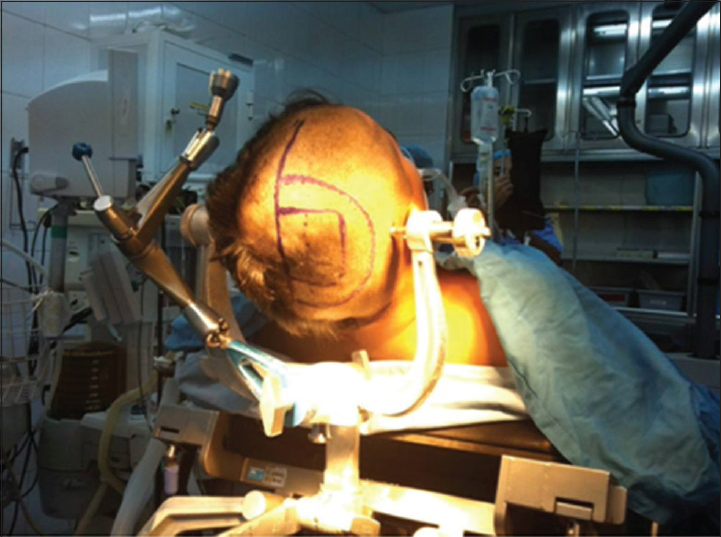
Surgery is the treatment of choice for symptomatic hydatid cysts, and Dowling–Orlando technique, which was used in our case, is the most widely accepted surgical technique. The patient was placed in the supine position and the head was held in a 45° flexed position using the Mayfield frame [
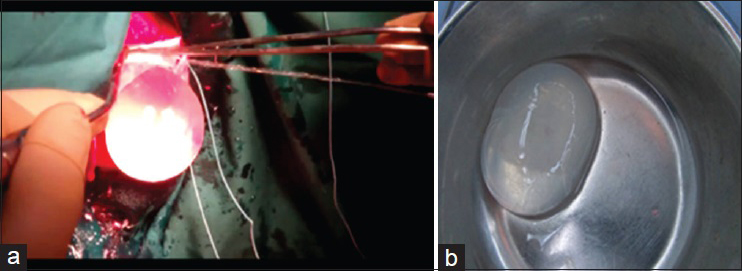
Neuronavigation was used to localize the cyst, and accordingly, a right parasagittal, inverted, C-shaped skin incision was planned overlying the lesion. Craniotomy was performed as usual and the dura was opened and reflected medially. Surgical patty was used to develop a plane between the cyst and the surrounding cortex, and then, a flexible catheter was inserted in between. The surgical field was then irrigated with hypertonic saline which applies gentle force between the cyst wall and brain parenchyma. With continuous irrigation, the cyst was delivered successfully without rupture in our case [
Figure 5
(a) Appearance of the hydatid cyst during removal by the Dowling-Orlando technique of hydrodissection. Injection of hypertonic saline dissected the cyst from the surrounding brain tissue, allowing the cyst to move outside of the brain. (b) Gross appearance of the hydatid cyst after intact removal
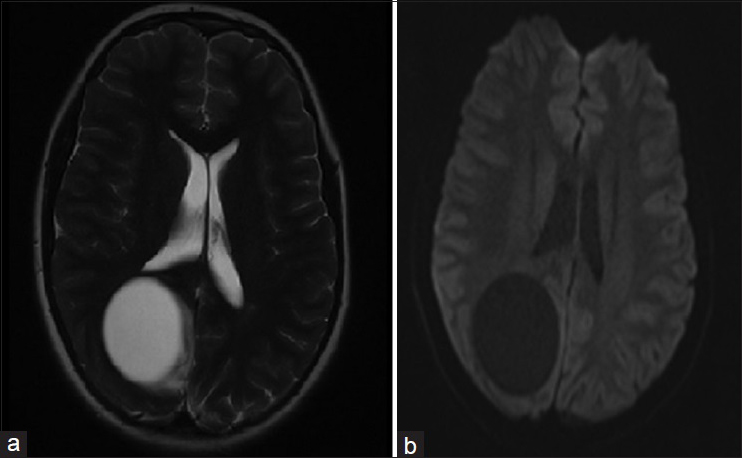
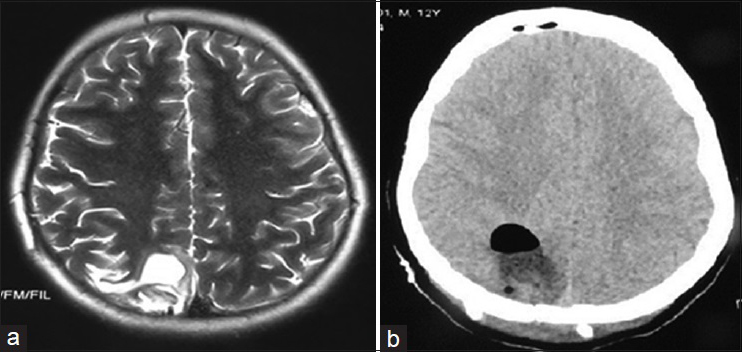
Postoperatively, the patient was transferred to surgical intensive care unit for observation. Subsequent physical examination showed complete resolution of the patient's signs and a dramatic improvement in his symptoms. Postoperative brain imaging documented the complete excision of the cyst and showed a small cystic cavity at the location of the excised cyst [
Figure 6
(a) Postoperative T2W axial brain MRI showing a small cystic cavity at the location of the excised cyst. (b) Postoperative brain CT without contrast documents the complete excision of the hydatid cyst and shows some postoperative changes such as pneumocephaly. There is no mass effect or midline shift
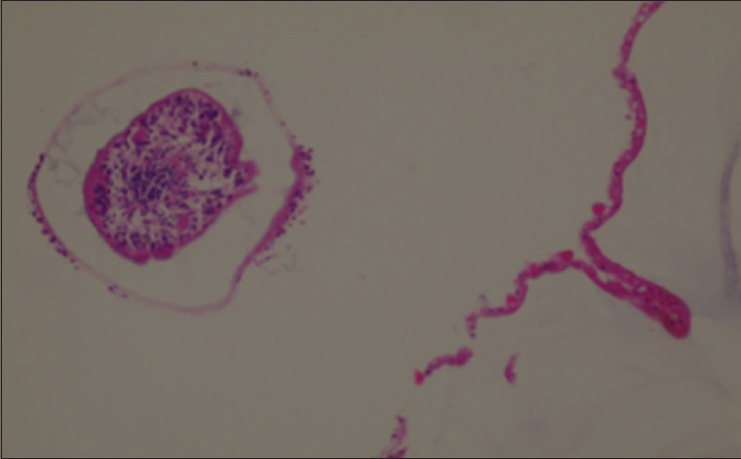
A specimen consisting of an intact hydatid cyst measuring around 5 × 5 cm and containing a clear fluid with a transparent surface was sent for histopathology. Microscopically, sections show a cyst wall composed of a laminated outer layer and an inner germinal cell layer with attached cysts containing scolices [
DISCUSSION
Hydatid cyst symptoms are usually due to elevated intracranial pressure (ICP) or mass effect on the surrounding structures. Headache and vomiting are almost always present along with bilateral papilledema.[
Detection of IgG anti-Echinococcus antibodies using enzyme-linked immunosorbent assay (ELISA) test is the definitive laboratory diagnosis. However, in isolated cerebral hydatid cyst, the antibody titers are usually low or absent, as in our case.
Superimposed bacterial infection, cyst rupture, and subsequent anaphylactic shock are the possible complications of hydatid disease. Intracranial hydatid cyst has a growth rate that ranges between 1.5 cm to 5.0 cm annually.[
Brain CT and MRI are not only important for radiological diagnosis but also for surgical planning.[
On MRI, the cyst appears hypointense on T1 weighted image and hyperintense with a hypointense halo around the cyst on T2 weighted images.[
For symptomatic hydatid cyst, surgery is the gold standard modality of treatment. The aim of the surgery is to deliver an intact cyst without rupture to prevent recurrence and anaphylactic reaction.[
A variety of surgical techniques are used for removal of the hydatid cyst.[
Intraoperative rupture of the cyst is the most common and most feared complication of surgery. Several precautions are usually added to avoid intraoperative cyst rupture, including: (1) Lowering the head-end of the patient which facilitates delivery of the cyst by gravity,[
Medical treatment can be a suitable alternative in asymptomatic and deep-seated lesions. The drug of choice is albendazole, usually for months to a year.[
Surgical removal, however, is still considered the treatment of choice for both symptomatic and asymptomatic intracranial hydatid lesions, because asymptomatic cysts may become symptomatic months after the initiation of the anti-helmenthic regimen.
The surgical technique and technical pitfalls
Preoperative
The anesthesia department should be aware of the risk of cyst rupture and be prepared for anaphylactic shock and dysautonomic disorders. In the case of high ICP, the anesthetist should also be prepared for sudden blood pressure decrease after craniotomy and durotomy.[
Image guidance for surgery
The dissection can be guided by microscopic dissection, especially for better visualization of adhesions between the cyst and the atrophic brain. The use of an endoscope is necessary for deep, ventricular, or spine lesions. Intraoperative MRI can guide the subcortical lesions and surrounding tissue.[
Dowling–Orlando's technique
The surgical approach depends on the location, size, and multiplicity of the hydatid cyst. The surgical approach using the classical Dowling's technique has the following surgical times: (1) Skin incision in large flaps, (2) craniotomy, (3) corticectomy of no less than three-fourth of the larger diameter of the cyst, (4) use of warm hypertonic saline (3%) in the surgical borders between the brain and the cyst, and (5) cyst delivery.[
For optimal surgical removal, the cyst should point to the upper part of the surgical plane and with a 30° head up The cyst should be in the center of the craniotomy and the burr holes should be made carefully with particular attention for eventual dura attachment to the bone to avoid pulling the dura with the cyst, which can lead to ruptures. Moreover, in cases of high ICP, the dura might be thinned Dura opening should be done circumferentially far from the cyst apex. Careful dissection should be done in special from the cyst apex that can be attached to the dura. Corticectomy should be done carefully with at least 3/4 of the cyst diameter Delicate dissection should be done with cottonoid in the borders and hypertonic saline continuous irrigation. Keep the cyst wall always moist to avoid rupture. The arachnoid dissection is preferably performed under microscope or surgical loupes Hypertonic saline should irrigate the cyst walls while the head can be slowly lowered to 45°. A soft rubber catheter can be used between the brain and cyst wall to irrigate the posterior wall of the cyst, which helps in the delivery of the cyst Careful attention should be given for the possible presence of adhesions between the cyst and the atrophied brain, which can hold the cyst wall during delivery. In case of cyst rupture, the sucker must be placed directly into the cyst for its aspiration.
Technical pitfalls
Contrast enhancement on the cyst wall can indicate an inflammatory process. The inflamed region had thinner walls, which are more susceptible for ruptures[ The atraumatic delivery of the unruptured cyst might be difficult in secondary infections due to the lack of clear borders[ Planning surgery for the head positioning is essential to ensure that the gravity helps in its delivery. After the corticectomy, the head of the operating table should be lowered At the corticectomy, bipolar can be used in the surrounding atrophic brain, and its dissection should be atraumatic with no cyst retraction.[ Warm saline is preferred and should be continuously applied through the borders, and after a good surgical plan, tubes can be gently introduced between brain and cyst wall to deliver saline directly to the posterior component[ Patties can be used for gentle retraction of the surrounding brain to release the borders from the cyst[ The surgical removal of large cysts should be slowly done because of possible rapid decompression syndrome, which can lead to dysautonomic disorders, hyperemia, and swelling in the surrounding brain.[
CONCLUSION
The incidence of primary intracranial hydatid cyst is very rare. It should, however, be considered as a differential diagnosis of cystic brain lesions, especially in children. Surgical removal of the cyst, using the Dowling technique, is the gold standard modality of treatment. Several precautions should be applied intraoperatively to avoid the catastrophe of cyst rupture.
Financial support and sponsorship
Department of Neurosurgery, Jordan University Hospital.
Conflicts of interest
There are no conflicts of interest.
Video Available on: www.surgicalneurologyint.com
References
1. Andronikou S, Welman CJ, Kader E. Classic and unusual appearances of hydatid disease in children. Pediatr Radiol. 2002. 32: 817-28
2. Bilge T, Batur S, Bilge S, Aydin Y, Aksoy B, Senol S. Primary multiple hydatid cysts of the brain: Case report. Surg Neurol. 1993. 39: 377-9
3. Boop FA, Jacobs RF, Young RL, Albright AL, Pollack IF, Adelson PD.editors. Brain abscess and encephalitis in children. Principles and Practice of pediatric Neurosurgery. New York: Thieme; 1999. p. 1203-26
4. Bukte Y, Kemanoglu S, Nazaroglu H, Ozkan U, Ceviz A, Simsek M. Cerebral hydatid disease: CT and MR imaging findings. Swiss Med Wkly. 2004. 134: 459-67
5. Carrea R, Dowling E, Guevera A. Surgical treatment of hydatid cysts of the central nervous system in the pediatric age (Dowling's technique). Childs Brain. 1975. 1: 4-21
6. Last accessed on 2013 Mar 11. Available at: http://www.cdc.gov/parasites/echinococcosis/epi.html.
7. Duishanbai S, Jiafu D, Guo H, Liu C, Liu B, Aishalong M. Intracranial hydatid cyst in children: Report of 30 cases. Childs Nerv Syst. 2010. 26: 821-7
8. El-Shamam O, Amer A, El-Atta MA. Magnetic resonance imaging of simple and infected hydatid cysts of the brain. Magn Reson Imaging. 2001. 19: 965-74
9. Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004. 17: 107-35
10. Greenberg MS. Handbook of Neurosurgery, Greenberg Graphics. 2010. p.
11. Gupta S, Desai K, Goel A. Intracranial hydatid cyst: A report of five cases and review of literature. Neurol India. 1999. 47: 214-7
12. Iniquez RA, Vinken PJ, Bruyn GW.editors. Echinococcus. Infection of the Nervous System. Hand Book of Clinical Neurology. Part 3. Amsterdam: Elsevier/North Holland Biomedical Press; 1978. p. 175-208
13. Izci Y, Tüzün Y, Seçer HI, Gönül E. Cerebral hydatid cysts: Techniques and pitfalls of surgical management. Neurosurg Focus. 2008. 24: E15-
14. Kiresi DA, Karabacakoglu A, Odev K, and Karakose S. Uncommon locations of hydatid cysts. Acta Radiol. 2003. 44: 622-36
15. Miabi Z, Hashemi HM, Ghaffarpour H, Ghelichna H, Media R. Clinicoradiological findings and treatment outcome in patients with intracranial hydatid cyst. Acta Med Iran. 2005. 43: 359-64
16. Ozkan U, Kemaloglu MS, Selçuki M. Gigantic intracranial mass of hydatid cyst. Childs Nerv Syst. 2001. 17: 623-5
17. Pedrosa I, Saiz A, Arrazola J, Ferreiros J, Pedrosa CS. Hydatid disease: Radiologic and pathologic features and complications. Radiographics. 2000. 20: 795-817
18. Tunaci M, Tunaci A, Engin G, Ozkorkmaz B, Ahishali B, Rozanes I. MRI of cerebral alveolar Echinococcosis. Neuroradiology. 1999. 41: 844-6
19. Tüzün M, Altinörs N, Arda IS, Hekimoğlu B. Cerebral Hydatid disease CT and MR findings. Clin Imaging. 2002. 26: 353-7
20. Tüzün M, Hekimoglu B. Hydatid disease of the CNS: Imaging features. AJR Am J Roentgenol. 1998. 711: 1497-500
21. Yurt A, Avci M, Selçuki M, Ozer F, Camlar M, Uçar K. Multiple cerebral hydatid cysts. Report of a case with 24 pieces. Clin Neurol Neurosurg. 2007. 109: 821-6