- Department of Neurosurgery, Neuroscience Institute, Hamad Medical Corporation, Doha, Qatar
- Department of Radiology, Neuroscience Institute, Hamad Medical Corporation, Doha, Qatar
- Department of Neurosurgery, University Medical Center, Mainz, Germany
Correspondence Address:
Ahmed Abd Elazim
Department of Neurosurgery, Neuroscience Institute, Hamad Medical Corporation, Doha, Qatar
DOI:10.4103/sni.sni_235_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Rasha Elbadry, Ahmed Abd Elazim, Kazim Mohamed, Mamdouh Issa, Ali Ayyad. Primary meningeal melanocytoma of the cerebellopontine angle associated with ipsilateral nevus of Ota: A case report. 04-Dec-2018;9:245
How to cite this URL: Rasha Elbadry, Ahmed Abd Elazim, Kazim Mohamed, Mamdouh Issa, Ali Ayyad. Primary meningeal melanocytoma of the cerebellopontine angle associated with ipsilateral nevus of Ota: A case report. 04-Dec-2018;9:245. Available from: http://surgicalneurologyint.com/surgicalint-articles/9107/
Abstract
Background:Cerebellopontine angle represents a complex anatomical area of the brain. A cerebellopontine angle lesion could be a vestibular schwannoma, meningioma, epidermoid cyst, or less likely, arachnoid cyst, metastasis, lower cranial nerves schwannoma, lipoma, hemangioma, paraganglioma, or vertebra-basilar dolichoectasia. Primary meningeal melanocytoma is a rare neoplasm, especially when it occurs at the cerebellopontine angle. Nevus of Ota (aka oculodermal melanocytosis) is a hyperpigmentation along the distribution of the ophthalmic and maxillary branches of trigeminal nerve; it occurs due to entrapment of melanocytes at the upper third of the dermis. It may not present at birth and may show up at puberty.
Case Description:We describe a case of primary meningeal melanocytoma of the cerebellopontine angle associated with nevus of Ota in a 46-year-old male patient presented with 7-day history of left arm weakness and vertigo. Computed tomography and MRI showed right-sided cerebellopontine angle mass, which was resected. Histopathology confirmed the meningeal melanocytic lesion and revealed its nature.
Conclusion:Primary meningeal melanocytoma of the brain is a rare but benign tumor; the association between meningeal melanocytoma and nevus of Ota is also rare and possibly explained by their common embryonic origin from neural crest cells. There are six cases reported so far in literature including our case for meningeal melanocytoma associated with nevus of Ota.
Keywords: Melanin, melanocytoma, meninges and cerebellopontine angle, nevus
INTRODUCTION
Cerebellopontine angle is considered a delicate anatomical region of the brain. A wide variety of cerebellopontine angle lesions exist. These could be a vestibular schwannoma, meningioma, epidermoid cyst, or less likely, arachnoid cyst, metastasis, lower cranial nerves schwannoma, lipoma, hemangioma, paraganglioma, or vertebra-basilar dolichoectasia.[
CASE REPORT
A 46-year- old Sri Lankan male patient without comorbidities presented with 1-week history of left arm weakness and vertigo of gradual onset and progressive course. He denied headache, vomiting, or convulsions. He had a history of nevus of Ota at the right side of the face. There is a positive family history of nevus of Ota.
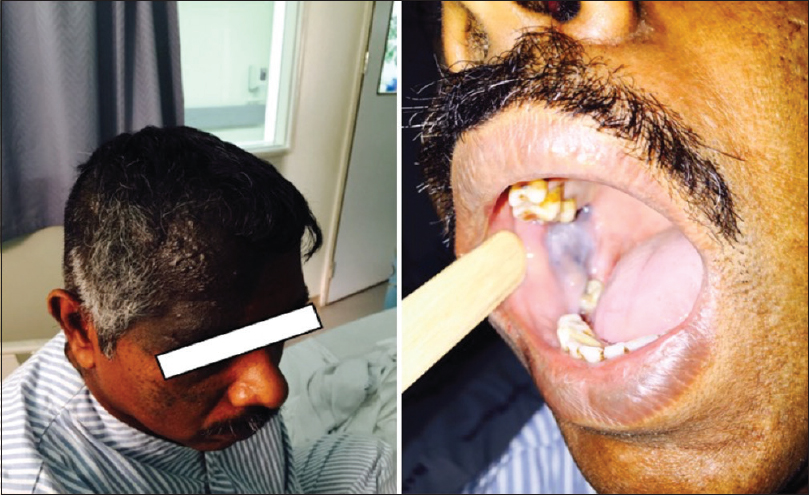
The patient was fully conscious and oriented, vitally stable, and has a bluish discoloration of the right frontal, periorbital region and buccal mucosa [
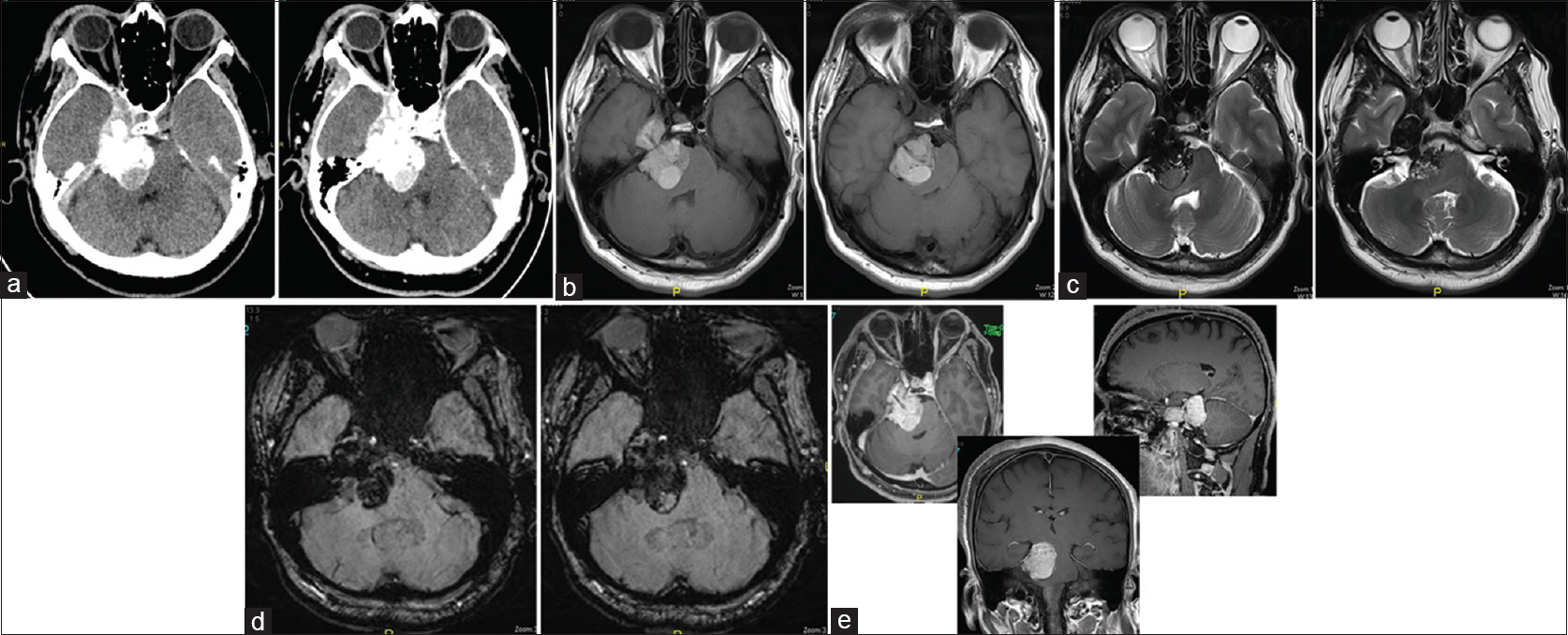
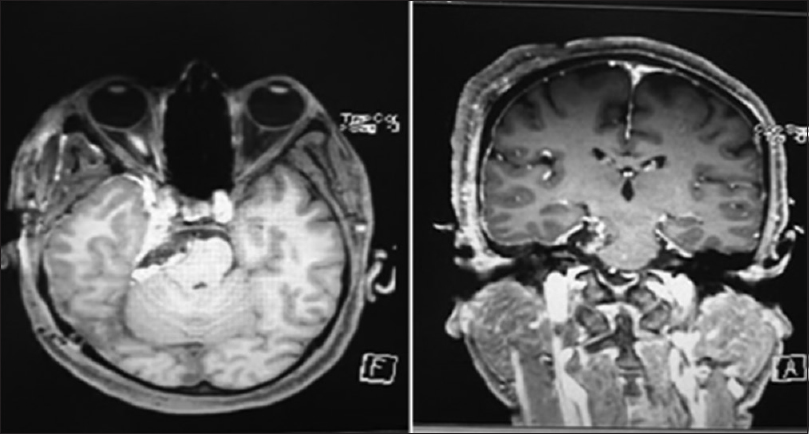
Computed tomography showed right-sided CP angle extra-axial mass lesion of mixed high and iso-attenuation value and showed faint postcontrast enhancement extending over the petrous apex to the right parasellar region medial to the right temporal lobe [
Figure 2
(a) Axial CT sections before and after IV contrast administration showing right-sided CP angle extra-axial mass lesion of mixed high and iso-attenuation value showing faint postcontrast enhancement extending over the petrous apex to the right parasellar region medial to the right temporal lobe. (b) Axial T1-weighted images showing lobulated intracranial extra-axial hyperintense lesion at the right CP angle and creeping along the petrous apex to the right parasellar region compressing the brain stem and indenting the medial aspect of the right temporal lobe. (c) Axial T2-weighted images showing the complex texture of the lesion with predominant low signal intensity and cystic areas of iso to high signal intensity. (d) Axial susceptibility-weighted images (SWI) showing ferromagnetic effects of the contents of the lesion with patchy areas of low signal intensity within a cystic lesion. (e) Axial, sagittal, and coronal contrast-enhanced T1-weighted images of the brain showing increase in the hyperintenisty of the lesion denoting its enhancement and showing the extension of the lesion from the right CP angle region to the right parasellar region with its mass effect on the brain stem and the right temporal lobe
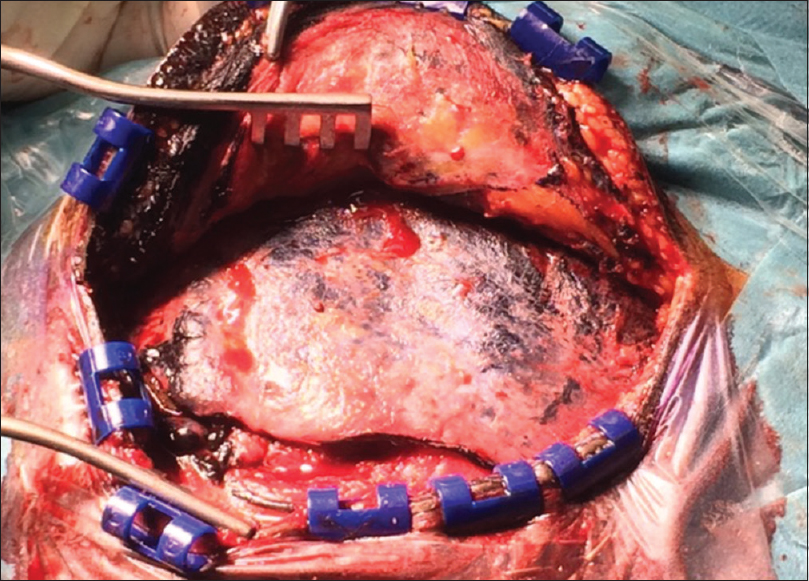
The patient underwent two-staged operations to resect the lesion utilizing two different approaches to secure proper resection. The first stage was through right retrosegmoid approach via 3-cm craniotomy. Facial, trigeminal, glossopharyngeal, and vagus nerves were visualized. There was a bluish lesion surrounding the trigeminal nerve. The part of the lesion that was accessible removed completely and part of it was resembling a hematoma. The second stage was through a right frontotemporal craniotomy; there was bluish discoloration of the skin, temporalis muscle, and dura [
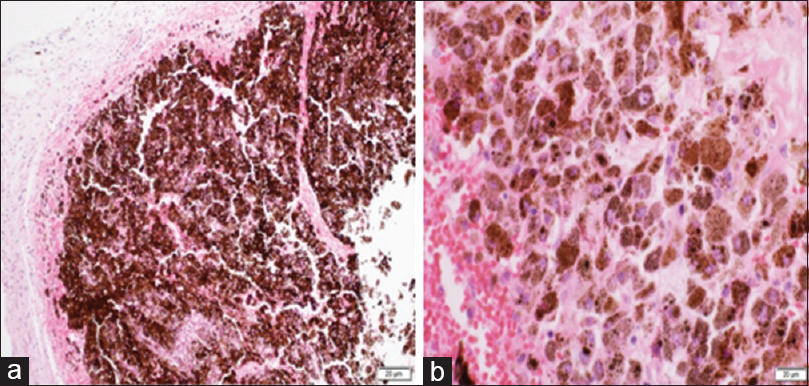
Meningeal nodules with heavy brownish pigmentation showed heavily pigmented melanocytes and S-100 positivity without atypia or necrosis concluding a meningeal melanocytoma [Figure
DISCUSSION
Nevus of Ota is a bluish nevus usually found unilaterally in the face involving branches 1 and 2 of the trigeminal nerve, appearing in the sclera, mucous membranes, and adjacent periorbital skin because of the presence of dermal melanocytes.[
Disorders that can be seen with nevus of Ota are Sturge–Weber syndrome, Klippel–Trenaunay syndrome, arteriovenous malformation, neurofibromatosis, Takayasu disease, and melanomas.[
Meningeal melanocytomas are benign-pigmented tumors arising from melanocytes; their incidence to occur in the brain is rare (0.06–0.1% of brain tumors) compared with melanomas.[
Clinical presentation of meningeal melanocytomas depends on the anatomical location and neurological structures compressed. Meningeal melanocytoma can present with intracerebral hemorrhage; Hino et al.[
According to the World Health Organization, melanotic neoplasms of the central nervous system are classified into melanocytosis, melanomatosis, melanocytoma, and malignant melanoma.[
Histopathologically, meningeal melanocytoma cells appear in sheets or bundles with occasional whorl appearance containing a high amount of melanin in the cytoplasm.[
Radiologically, meningeal melanocytoma appears homogenously hyperintense on T1-weighted images with contrast enhancement by gadolinium and hypointense on T2-weighted images owing to its melanin content.[
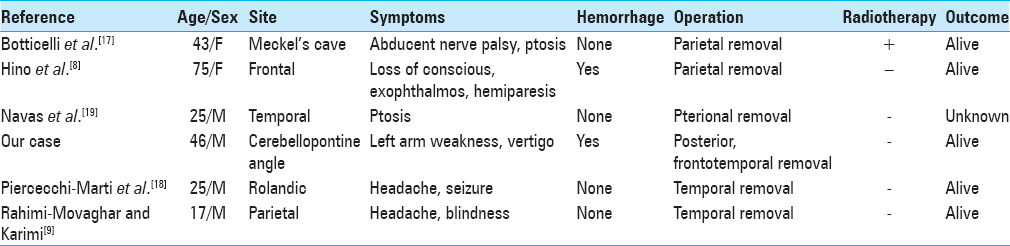
In the 95 case series of Rahimi-Movaghar and Karimi,[
The association between meningeal melanocytoma and nevus of Ota is rare, based on our review of the literature; there are six cases of meningeal melanocytoma associated with nevus of Ota including our case [
CONCLUSION
Primary meningeal melanocytoma of the brain is a rare but benign tumor; the association between meningeal melanocytoma and nevus of Ota is also rare and possibly explained by their common embryonic origin from neural crest cells. There are six cases reported so far in literature including our case for meningeal melanocytoma associated with nevus of Ota.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Botticelli AR, Villani M, Angiari P, Peserico L. Meningeal melanocytoma of Meckel's cave associated with ipsilateral Ota's nevus case report. Cancer. 1983. 51: 2304-10
2. Chan HH, Kono T. Nevus of Ota: Clinical aspects and management. Skinmed. 2003. 2: 89-98
3. Chow M, Clarke DB, Maloney WJ, Sangalang V. Meningeal melanocytoma of the planum sphenoidale: Case report and review of the literature. J Neurosurg. 2001. 94: 841-5
4. Hidano A, Kajima H, Ikeda M, Miyasato H, Niimura M. Natural history of nevus of Ota. Arch Dermatol. 1967. 95: 187-95
5. Hino K, Nagane M, Fujioka Y, Shiokawa Y. Meningeal melanocytoma associated with ipsilateral nevus of Ota presenting as intracerebral hemorrhage: Case report. Neurosurgery. 2005. 56: E1376-
6. Jaiswal S, Jaiswal AK, Vij M, Behari S, Pandey R. Primary meningeal melanocytoma of cerebellopontine angle: A case report with 12 years follow up. Basic Appl Pathol. 2011. 4: 99-102
7. Kawaguchi T, Kawano T, Kazekawa K, Nakashima S, Honma T, Kaneko Y. Meningeal melanocytoma in the left frontal region. Brain Tumor Pathol. 1998. 15: 58-62
8. Kopf A. Weidman AI: Nevus of Ota. Arch Dermatol. 1962. 85: 75-88
9. Kurita H, Segawa H, Shin M, Ueki K, Ichi S, Sasaki T. Radiosurgery of meningeal melanocytoma. J Neurooncol. 2000. 46: 57-61
10. Maize J, Ackerman B.editorsPigmented Lesions of the Skin. Philadelphia: Bernard, Lea and Febiger; 1987. p. 73-162
11. Navas M, Pascual JM, Fraga J, Pedrosa M, Shakur S, Carrasco R. Intracranial intermediate-grade meningeal melanocytoma with increased cellular proliferative index: An illustrative case associated with a nevus of Ota. J Neurooncol. 2009. 95: 105-15
12. Ota M. Nevus fusco-caeruleus ophthalmomaxillaris. Jpn J Dermatol. 1939. 46: 369-72
13. Piercecchi-Marti MD, Mohamed H, Liprandi A, Gambarelli D, Grisoli F, Pellissier JF. Intracranial meningeal melanocytoma associated with ipsilateral nevus of Ota: Case report. J Neurosurg. 2002. 96: 619-23
14. Rades D, Heidenreich F, Tatagiba M, Brandis A, Karstens JH. Therapeutic options for meningeal melanocytoma: Case report. J Neurosurg. 2001. 95: 225-31
15. Rahimi-Movaghar V, Karimi M. Meningeal melanocytoma of the brain and oculodermal melanocytosis (nevus of Ota): Case report and literature review. Surg Neurol. 2003. 59: 200-10
16. Rapini RP, Bolognia JL, Jorizzo JL.editorsDermatology: 2-Volume Set. St. Louis: Mosby; 2007. p.
17. Stuart C. Naevus of Ota. Br J Dermatol. 1955. 67: 317-9
18. Uematsu Y, Yukawa S, Yokote H, Itakura T, Hayashi S, Komai N. Meningeal melanocytoma: Magnetic resonance imaging characteristics and pathological features: Case report. J Neurosurg. 1992. 76: 705-10
19. Winston KR, Sotrel A, Schnitt SJ. Meningeal melanocytoma: case report and review of the clinical and histological features. J Neurosurg. 1987. 66: 50-7