- Department of Neurosurgery, Zucker School of Medicine at Hofstra/Northwell, Manhasset, New York, USA
- Department of Pathology and Lab Medicine, Zucker School of Medicine at Hofstra/Northwell, Manhasset, New York, USA
- Department of Neuroradiology, Zucker School of Medicine at Hofstra/Northwell, Manhasset, New York, USA
- Department of Otolaryngology and Communicative Disorders, Zucker School of Medicine at Hofstra/Northwell, Manhasset, New York, USA
Correspondence Address:
Julia R. Schneider
Department of Neurosurgery, Zucker School of Medicine at Hofstra/Northwell, Manhasset, New York, USA
DOI:10.4103/sni.sni_483_17
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Julia R. Schneider, Kevin Kwan, Kay O. Kulason, Lukas J. Faltings, Stephanie Colantonio, Scott Safir, Tina Loven, Jian Yi Li, Karen S. Black, B. Todd Schaeffer, Mark B. Eisenberg. Primary solitary retro-clival amyloidoma. 15-May-2018;9:100
How to cite this URL: Julia R. Schneider, Kevin Kwan, Kay O. Kulason, Lukas J. Faltings, Stephanie Colantonio, Scott Safir, Tina Loven, Jian Yi Li, Karen S. Black, B. Todd Schaeffer, Mark B. Eisenberg. Primary solitary retro-clival amyloidoma. 15-May-2018;9:100. Available from: http://surgicalneurologyint.com/surgicalint-articles/primary-solitary-retro%e2%80%91clival-amyloidoma/
Abstract
Background:Amyloidosis encompasses a group of disorders sharing the common feature of intercellular deposition of amyloid protein by several different pathogenetic mechanisms. Primary solitary amyloidosis, or amyloidoma, is a rare subset of amyloidosis in which amyloid deposition is focal and not secondary to a systemic process or plasma cell dyscrasia.
Case Description:This 84-year-old female presented with history of multiple syncopal episodes, dysphagia, and ataxia. Motor strength was 3+/5 in the right upper extremity. Rheumatoid factor, cyclic citrullinated peptide (CCP), and anti-nuclear antibody (ANA) were normal. Serum and urine immune-electrophoresis detected no abnormal bands. Computed tomography (CT) and magnetic resonance imaging (MRI) demonstrated a non-enhancing soft-tissue mass extending from the retro-clivus to C2 posteriorly, eccentric to the right with severe mass effect on the upper cervical medullary junction. Endoscopic trans-nasal debulking of the retro-clival mass was performed with occiput to C5 posterior instrumentation for spinal stabilization.
Conclusions:Primary solitary amyloidosis, unlike other forms of amyloidosis, has an excellent prognosis with local resection. Diagnosis requires special stains and a degree of suspicion for the disease. This is the first report to document an endoscopic trans-nasal approach for removal of a primary solitary amyloidosis of the retro-clivus. Management of vertebral amyloidoma involves aggressive local resection of the tumor when feasible and spine stabilization as the degree of tumor involvement mandates. Complete evaluation for the diagnosis of systemic amyloidosis is essential for the management and prognostication. Surgeons encountering such lesions must maintain high suspicion for this rare disease and advise pathologists accordingly to establish the correct diagnosis.
Keywords: Amyloidoma, cervical instrumentation, cervical spine, primary solitary amyloidosis
INTRODUCTION
The term amyloidosis encompasses a group of disorders that have as their common feature the intercellular deposition of the fibrillary protein, amyloid, by several different pathogenic mechanisms.[
CASE REPORT
Presentation
An 84-year-old right-handed female with a past medical history of paroxysmal atrial fibrillation, congestive heart failure, and colon resection presented with a several-month history of progressive ataxia and dysphagia. She had been at a rehabilitation center for the past month due to multiple syncopal episodes. At initial presentation the patient was able to ambulate only short distances with a walker. She also described some difficulty with fine motor skills with her hands. Her family states that she had been able to ambulate as recently as several months ago. She denied any additional constitutional symptoms including fever, chills, or nausea. She denied neck pain. She had not undergone any spine procedures previously. Her preoperative modified Rankin score (mRS) was 3.
Examination
On physical examination motor strength was 3+/5 in the right upper extremity. The remainder of the neurologic and general examination was unremarkable. Rheumatoid factor, cyclic citrullinated peptide (CCP), and ANA were normal. The serum and urine immune-electrophoresis detected no abnormal bands.
Imaging
Computed tomography (CT) and magnetic resonance imaging (MRI) were performed and showed a non-enhancing soft-tissue mass extending superior from the retro-clivus to C2 posteriorly, eccentric to the right with severe mass effect on the upper cervical medullary junction [Figures
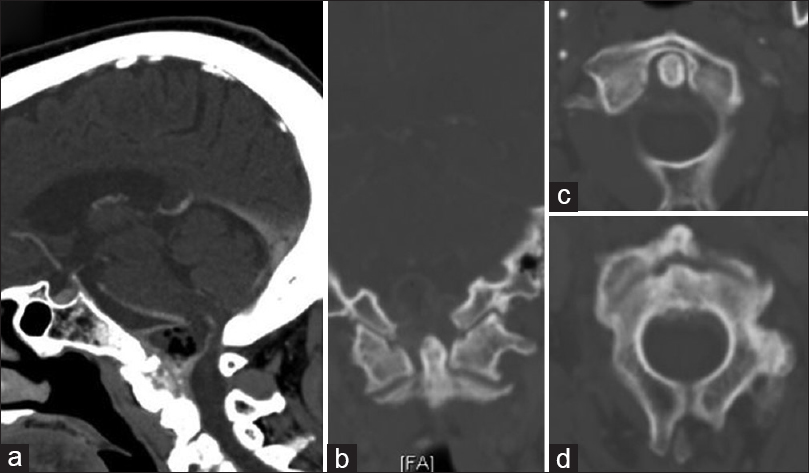
Figure 1
Initial sagittal (a) and coronal (b) computed tomography scan obtained at presentation demonstrate a non-enhancing mass centered along the posterior aspect of the clivus and C2, eccentric to the right, with soft tissue air. Not associated with erosive bony changes of either the dens (c) or the body of C2 (d)
On CT, amyloidomas generally are hyperattenuating and enhance following contrast administration.[
On MRI, amyloidoma signal characteristics are variable. T1 and T2 images range from hypointense to hyperintense. There is vivid contrast enhancement on T1-contrast images, and peripheral radial enhancement may be observed. Additionally, on T2 susceptibility weighted images (SWI), microhemorrhages may be observed.[
Surgery
The decision was made to proceed with operative intervention for decompression of her cervical spinal cord and to obtain tissue for diagnostic pathologic examination. As the amyloid tumor arose posterior to C2 and extended caudally to the retro-clivus, we decided on an endoscopic trans-nasal approach with combined posterior stabilization. A transoral approach was considered, but ultimately, was not chosen because of the retroclival nature of the lesion. Although a transoral approach can provide a wider surgical field, the trans-nasal approach allowed better visualization of the clivus as well as structures posterior to C1 better.[
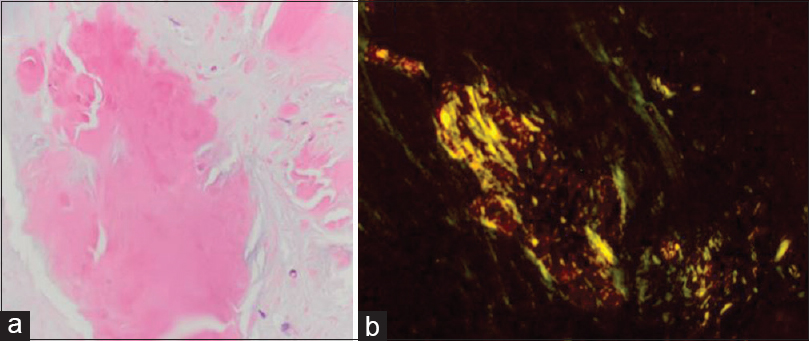
Histopathology
Multiple specimens of the lesion were taken and underwent pathologic examination. Microscopic examination revealed amorphous, eosinophilic deposits. Special stains revealed focally strong congophilia with Congo red stain for amyloid, consistent with cerebral amyloidoma.[
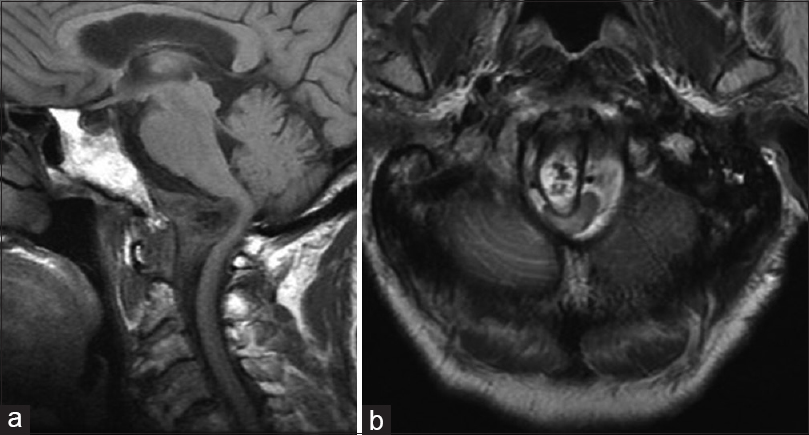
Figure 3
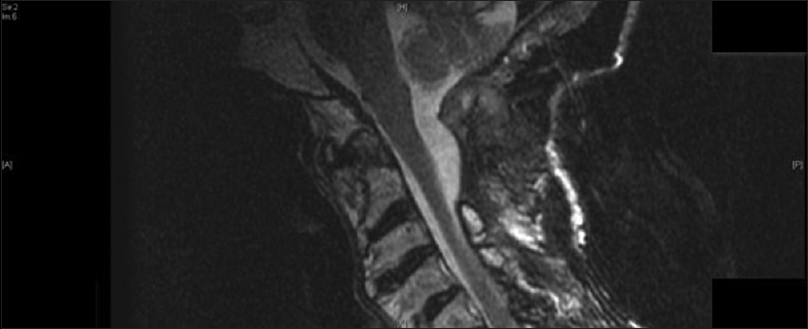
Postoperative T1-weighted sagittal (a) and axial (b) magnetic resonance imaging demonstrate interval postoperative changes including partial debulking of the mass and evidence of the fat graft. There was improvement of the cord kinking at the cervical medullary junction, but persistent moderate canal stenosis. Lateral x-ray films demonstrate a stable construct (c)
Postoperative course
The patient underwent monitoring in the neurosurgical intensive care unit and gradual tapering of her lumbar drain. Extensive systemic evaluation did not reveal any inflammatory processes, systemic amyloidosis, or plasma cell dyscrasia. Serum rheumatoid factor, CCPs, and ANA were negative. Urine and serum protein electrophoresis and immune-electrophoresis did not reveal a monoclonal gammopathy. The patient was discharged to subacute rehabilitation 2 weeks postoperatively with improved neuro-motor exam. MRI imaging revealed debulking of the retro-clival mass with improvement of cervical medullary kinking. CT imaging demonstrated a stable spine construct [
DISCUSSION
Amyloidosis is a disease complex resulting from the extracellular deposition of amyloid, an insoluble proteinaceous material with a beta-pleated sheet configuration.[
Amyloidosis may occur spontaneously (primary amyloidosis) or in response to chronic disease processes (reactive/secondary amyloidosis). The WHO-IUIS nomenclature subcommittee classified amyloid and amyloidosis into 15 types based on the amino acid sequence of the specific amyloid protein involved in each disease type.[
Most cerebral amyloidomas are AL type resulting from immunoglomulin-derived kappa and lambda light chains, which are likely made from plasma cells.[
However, by definition, AL type is associated with plasma cell dyscrasia and is detectable in almost all patients with AL type.[
Prognosis for patients with amyloidosis is dependent on the specific form of amyloidosis. In secondary amyloidosis, treatment of the underlying disease can slow or reverse the progression of amyloid disease with 5- and l0-year survival after diagnosis not uncommon. Patients with immunocytic-derived amyloidosis have the worst prognosis with less than 1-year survival.[
Skeletal amyloid deposits often have an associated soft-tissue mass that may contain variable amounts of calcification.[
Primary amyloidosis is characterized by the lack of detectable plasma cell dyscrasia or abnormal serum proteins.[
Potential management options reported in the literature include non-invasive approaches utilizing chemotherapy, surgical approaches aimed toward as complete a resection as possible of the tumor with stabilization of the spine.[
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aiken AH, Akgun H, Tihan T, Barbaro N, Glastonbury C. Calcifying pseudoneoplasms of the neuraxis: CT, MR imaging, and histologic features. AJNR Am J Neuroradiol. 2009. 30: 1256-60
2. Cohen AS.editorsHarrison's principles of internal medicine. New York: NY: McGraw Hill; 1994. Vol. 2: 1625-30
3. Dähnert W.editorsRadiology review manual. Lippincott Williams & Wilkins; 2003. p.
4. Dickman CA, Sonntag VK, Johnson P, Medina M. Amyloidoma of the cervical spine: A case report. Neurosurgery. 1988. 22: 419-22
5. Farrell K, Stobo DB, Soutar R. Cervical amyloidoma successfully treated with bortezomib and dexamethasone. J Clin Oncol. 2011. 29: e512-3
6. Fischer B, Palkovic S, Rickert C, Weckesser M, Wassmann H. Cerebral AL lambda-amyloidoma: Clinical and pathomorphological characteristics. Review of the literature and of a patient. Amyloid. 2007. 14: 11-9
7. García Duque S, Medina Lopez D, Ortiz de Méndivil A, Diamantopoulos Fernández J. Calcifying pseudoneoplasms of the neuraxis: Report on four cases and review of the literature. Clin Neurol Neurosurg. 2016. 143: 116-20
8. Glenner GG.editorsInternal medicine. St Louis, MO: Mosby Year-Book Inc; 1994. p. 2511-4
9. Greenberg MSHandbook of Neurosurgery. 2010. p.
10. Heß K, Purrucker J, Hegenbart U, Brokinkel B, Berndt R, Keyvani K. Cerebral amyloidoma is characterized by B-cell clonality and a stable clinical course. Brain Pathol. 2018. 28: 234-9
11. Hwang SS, Park YH, Kim JY, Jung SL, Ahn MI, Park CK. Primary amyloidoma of the cervical spine. AJNR Am J Neuroradiol. 2000. 21: 601-3
12. Iplikcioglu AC, Bek S, Gokduman CA, Cosar M, Sav A. Primary solitary cervical amyloidosis: Case report and review of the literature. Spine. 2007. 32: E45-7
13. Jack CR, Reese DF, Scheithauer BW. Radiographic findings in 32 cases of primary CNS lymphoma. AJR Am J Roentgenol. 1986. 146: 271-6
14. Jahnke K, Schilling A, Heidenreich J, Stein H, Brock M, Thiel E. Radiologic morphology of low-grade primary central nervous system lymphoma in immunocompetent patients. AJNR Am J Neuroradiol. 2005. 26: 2446-54
15. Johnson BA, Fram EK, Johnson PC, Jacobowitz R. The variable MR appearance of primary lymphoma of the central nervous system: Comparison with histopathologic features. AJNR Am J Neuroradiol. 1997. 18: 563-72
16. Khalighi MA, Dean Wallace W, Palma-Diaz MF. Amyloid nephropathy. Clin Kidney J. 2014. 7: 97-106
17. Laeng RH, Altermatt HJ, Scheithauer BW, Zimmermann DR. Amyloidomas of the nervous system: A monoclonal B-cell disorder with monotypic amyloid light chain lambda amyloid production. Cancer. 1998. 82: 362-74
18. Landau D, Avgeropoulos N, Ma J. Cerebral amyloidoma mimicking intracranial tumor: A case report. J Med Case Rep. 2010. 4: 308-
19. Miller-Thomas MM, Sipe AL, Benzinger TL, McConathy J, Connolly S, Schwetye KE. Multimodality review of amyloid-related diseases of the central nervous system. Radiographics. 2016. 36: 1147-63
20. Moonis G, Savolaine ER, Anvar SA, Khan A. MRI findings of isolated beta-2 microglobulin amyloidosis presenting as a cervical spine mass. Case report and review of the literature. Clin Imaging. 1999. 23: 11-4
21. Mulleman D, Flipo RM, Assaker R, Maurage CA, Chastanet P, Ducoulombier V. Primary amyloidoma of the axis and acute spinal cord compression: A case report. Eur Spine J. 2004. 13: 244-8
22. Mullins , KJ , Meyers SP, Kazee AM. Primary solitary amyloidosis of the spine: A case report and review of the literature. Surg Neurol. 1997. 48: 405-8
23. Porchet F, Sonntag VK, Vrodos N. Cervical amyloidoma of C2.Case report and review of the literature. Spine. 1998. 23: 133-8
24. Seker A, Inoue K, Osawa S, Akakin A, Kilic T, Rhoton AL Jr. Comparison of endoscopic transnasal and transoral approaches to the craniovertebral junction. World Neurosurg. 2010. 74: 583-602
25. Sisk AE, Castro FH, Palma-Diaz MF, Lassman CR, Wallace WD. Kappa restricted (AL) amyloidosis demonstrates strong PAS and JMS staining compared to lambda restricted and AA type amyloidosis. J Clin Nephrol Res. 2017. 4: 1056-
26. Toh CH, Wei KC, Chang CN, Hsu PW, Wong HF, Ng SH. Differentiation of pyogenic brain abscesses from necrotic glioblastomas with use of susceptibility-weighted imaging. AJNR Am J Neuroradiol. 2012. 33: 1534-8
27. Werner BC, Shen FH, Shimer AL. Primary cervical amyloidoma: A case report and review of the literature. Spine J. 2013. 13: e1-e7
28. Yoshida A, Borkar S, Singh B, Ghossein RA, Schöder H. Incidental detection of concurrent extramedullary plasmacytoma and amyloidoma of the nasopharynx on [18F] fluorodeoxyglucose positron emission tomography/computed tomography. J Clin Oncol. 2008. 26: 5817-9