- Department of Neurosurgery, Catholic University, Rome, Italy
- Department of Pathology, Catholic University, Rome, Italy
Correspondence Address:
Nicola Montano
Department of Pathology, Catholic University, Rome, Italy
DOI:10.4103/sni.sni_141_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Nicola Montano, Alessandro Rapisarda, Fabrizio Pignotti, Marco Gessi. Prognostic factors in brain metastases from laryngeal squamous cell carcinoma: Case report and review. 04-Sep-2018;9:179
How to cite this URL: Nicola Montano, Alessandro Rapisarda, Fabrizio Pignotti, Marco Gessi. Prognostic factors in brain metastases from laryngeal squamous cell carcinoma: Case report and review. 04-Sep-2018;9:179. Available from: http://surgicalneurologyint.com/surgicalint-articles/8992/
Abstract
Background:Brain metastases from laryngeal squamous cell carcinoma (SCC) are rare, and there are no standardized treatments. Here we reported on a case of brain metastasis from laryngeal SCC and performed a literature review on these cases. Moreover, by plotting Kaplan–Meier curves, we carried out a survival analysis to provide an estimation of overall survival (OS) and to find possible prognostic factors.
Case Description:A 65-year-old male was admitted to our department with a large left occipital lesion. Three years ago, the patient had undergone total laryngectomy with bilateral neck dissection with a diagnosis of a poor differentiated SCC. The occipital lesion was totally removed. A diagnosis of a brain metastasis from laryngeal SCC was made. The patient was submitted to adjuvant chemotherapy and radiation therapy. He is in good clinical conditions at 7-month follow-up with a still ongoing chemotherapy. From survival analysis, we have found that surgery and/or radiochemotherapy increase the OS of these patients compared with untreated cases. Moreover, Karnofsky performance status (KPS) score ≥70 and recursive partitioning analysis (RPA) classes I and II were associated with better OS in these patients.
Conclusion:Brain metastases from laryngeal SCC are rare. This is the first study in which a survival analysis of these cases has been performed. Surgery and/or radio-chemotherapy increase the survival of these patients compared with untreated cases. Moreover, KPS score and RPA class affect the outcome of these patients.
Keywords: Brain metastasis, laryngeal squamous cell carcinoma, literature review, overall survival, prognosis
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) can arise from the oral cavity, oropharynx, hypopharynx, larynx, and nasopharynx. There are well-known risk factors for these tumors such as sustained exposure to tobacco and tobacco-like products, cigarette smoking, and alcohol abuse. Moreover, exposure to high-risk oncogenic human papillomavirus increases the risk of development of oropharyngeal SCC. Overall, about 644,000 new cases each year are reported worldwide.[
CASE REPORT
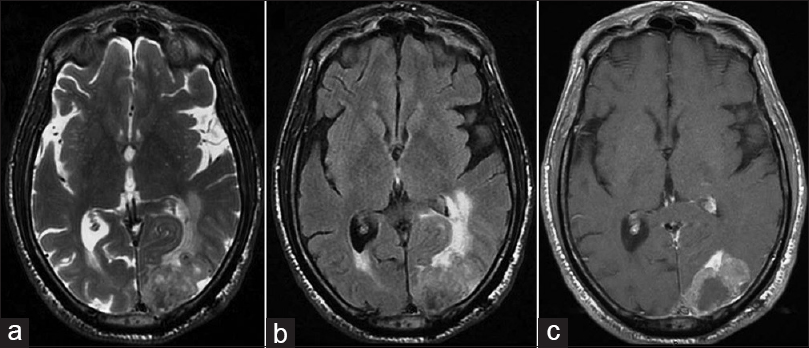
A 65-year-old male was admitted to our department with a right hemiparesis. Three years ago, the patient had undergone total laryngectomy with bilateral neck dissection. He had been a smoker for 40 years before that operation. On histopathology a diagnosis of a poor differentiated SCC (T4a, N2c, M0; G3) had been made. The patient had then been submitted to adjuvant radiation therapy. One year later, a total body computed tomography (CT) scan detected multiple pulmonary lesions with mediastinal lymph node enlargement, which were treated with chemotherapy (carboplatin and fluorouracil, but the treatment was interrupted after only two sessions for drug toxicity). Radiological follow-up showed stability of pulmonary lesions for 18 months when a disease progression was evidenced by a lung CT scan. Thus, the patient underwent a second cycle of chemotherapy with paclitaxel (interrupted after three sessions for the onset of fatigue and hand–foot syndrome) with stability of pulmonary lesions. Due to the onset of the right hemiparesis, the patient was submitted to brain magnetic resonance imaging, which showed a large left occipital mass with contrast enhancement and perilesional edema [
Figure 1
Brain magnetic resonance imaging showing a polylobate lesion in the left occipital lobe isohypointense on (a) axial T2-weighted images and (b) fluid attenuation inversion recovery sequences. Perilesional edema is evident with compression of the occipital horn of the lateral ventricle and mass effect. On (c) T1-weighted sequences after gadolinium administration, the tumor showed a dishomogeneous contrast enhancement
Figure 2
Histopathological examination revealed a metastasis of a poorly differentiated carcinoma composed of nests of cell with hyperchromic and irregular nuclei (a and b). The tumor showed strong immunoreactivity for pan-cytokeratins (AE1/AE3) and focal positivity for CK5/6 and p40, showing areas of squamous differentiation (c and d)
DISCUSSION
SCC represents more than 90% of the tumors arising from the larynx, and smoking habit and alcohol consumption are reported as the most important risk factors for its development.[
Figure 3
Kaplan–Meier survival curve of patients with intracranial metastasis from laryngeal squamous cell carcinoma stratified by treatment. (a) There was a statistically significant difference in the overall survival between the treated and untreated groups. (b) KPS score ≥70 and (c) RPA classes I and II were associated with better overall survival
It should be evidenced that our study has some limitations such as the limited number of cases included in the analysis and the heterogeneity of considered studies. All these factors should be considered as potential bias in survival analysis. Nonetheless, in our opinion, this study has the merit of providing indications about the survival and prognosis of patients with a brain metastasis from laryngeal SCC. In summary, the prognosis of an untreated brain metastasis from laryngeal SCC is very poor. Surgery and/or radiochemotherapy increase the survival of these patients. KPS score and RPA class are confirmed as prognosticators in these patients.
CONCLUSION
Brain metastases from laryngeal SCC are rare. No standardized treatments are available in the literature. This is the first study that, carrying out a survival analysis of previous cases, has provided indications about the survival and prognosis of these patients. Surgery and/or radiochemotherapy increase the survival of these patients compared with untreated cases. Moreover, KPS score and RPA class affect the outcome of these patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understand, that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abramson AL, Parisier SC, Zamansky MJ, Sulka M. Distant metastases from carcinoma of the larynx. Laryngoscope. 1971. 81: 1503-11
2. Ahmad K, Kim YH, Post MJ, Byun Y, Fayos JV. Hematogenous neoplastic spread to the cavernous sinus: Report of a case. Int J Radiat Oncol Biol Phys. 1984. 10: 321-
3. Altay T, Krisht KM, Couldwell WT. Sellar and parasellar metastatic tumors. Int J Surg Oncol 2012. 2012. p.
4. de Bree R, Mehta DM, Snow GB, Quak JJ. Intracranial metastases in patients with squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg. 2001. 124: 217-21
5. Dimri K, Rastogi N, Lal P. Intracranial metastasis in carcinoma of the glottis. Lancet Oncol. 2003. 4: 515-6
6. Ghosh-Laskar S, Agarwal JP, Yathiraj PH, Tanawade P, Panday R, Gupta T. Brain metastasis from nonnasopharyngeal head and neck squamous cell carcinoma: A case series and review of literature. J Cancer Res Ther. 2016. 12: 1160-3
7. Leemans CR, Tiwari R, Nauta JJ, van der Waal I, Snow GB. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer. 1993. 71: 452-6
8. Marur S, Forastiere AA. Head and neck squamous cell carcinoma: Update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016. 91: 386-96
9. Merino OR, Lindberg RD, Fletcher GH. An analysis of distant metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1977. 40: 145-51
10. Pan Z, Yang G, Qu L, Yuan T, Pang X, Wang Y. Leptomeningeal metastasis from early glottic laryngeal cancer: A case report. Oncol Lett. 2015. 10: 2915-8
11. Raitiola H, Pukander J, Laippala P. Glottic and supraglottic laryngeal carcinoma: Differences in epidemiology, clinical characteristics and prognosis. Acta Otolaryngol. 1999. 119: 847-51
12. Traserra J, Comas J, Conde C, Cuchi A, Cardesa A. Metastatic involvement of the cavernous sinus from primary pharyngolaryngeal tumors. Head Neck. 1990. 12: 426-9
13. Uzal MC, Kocak Z, Doganay L, Tokatli F, Caloglu M, Kilincer C. Pituitary metastasis mimicking a macroadenoma from carcinoma of the larynx: A case report. Tumori. 2001. 87: 451-4
14. Warwick-Brown NP, Cheesman AD. Intracranial metastases from a supraglottic carcinoma (a case report). J Laryngol Otol. 1987. 101: 624-6
15. Weiss R, Myssiorek D, Kahn L, Patel M. Laryngeal squamous cell carcinoma metastatic to the pituitary gland: A case study. Otolaryngol Head Neck Surg. 1994. 111: 816-9
16. Zahra M, Tewfik HH, McCabe BF. Metastases to the cavernous sinus from primary carcinoma of the larynx. J Surg Oncol. 1986. 31: 69-70