- Department of Tropical Diseases and Imaging Diagnosis, School of Medicine, University of São Paulo State, São Paulo, Brazil
- Department of Neurology and Neurosurgery, School of Medicine, University of São Paulo State, São Paulo, Brazil
- Department of Pathology, School of Medicine, University of São Paulo State, São Paulo, Brazil
Correspondence Address:
Seizo Yamashita
Department of Pathology, School of Medicine, University of São Paulo State, São Paulo, Brazil
DOI:10.4103/2152-7806.206006
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Luiz Antonio Jorge, Seizo Yamashita, André Petean Trindade, Luiz Antonio Lima Resende, Marco Antonio Zanini, José Cândido Caldeira Xavier, Marcelo Padovani de Toledo Moraes. Pseudotumoral neuroparacoccidioidomycosis of the posterior fossa: A case report and review of the literature. 10-May-2017;8:76
How to cite this URL: Luiz Antonio Jorge, Seizo Yamashita, André Petean Trindade, Luiz Antonio Lima Resende, Marco Antonio Zanini, José Cândido Caldeira Xavier, Marcelo Padovani de Toledo Moraes. Pseudotumoral neuroparacoccidioidomycosis of the posterior fossa: A case report and review of the literature. 10-May-2017;8:76. Available from: http://surgicalneurologyint.com/surgicalint-articles/pseudotumoral-neuroparacoccidioidomycosis-of-the-posterior-fossa-a-case-report-and-review-of-the-literature/
Abstract
Background:Paracoccidioidomycosis is a systemic mycosis of significant importance in some Latin American countries. The widespread use of neuroimaging methods has shown that involvement of the central nervous system was more frequent than previously reported. The most common form of occurrence of neuroparacoccidioidomycosis is the pseudotumoral one. The authors report a case of pseudotumoral neuroparacoccidioidomycosis localized in the posterior fossa.
Case Description:A 49-year-old single man, rural worker, born and raised in Laranjal Paulista-SP, was admitted to the hospital with 3 months history of bilateral occipital headache every day. Along with a history of active smoking and previous use of alcohol, the patient reported personal history of mild occipitotemporal injury 3 months ago. The patient was submitted to computed tomography in a 16-row multidetector scanner, which revealed a nodular hypodense lesion with a ring-enhancement and associated perilesional edema in the left cerebellar hemisphere. Radiological workup was initiated to investigate the eventual primary neoplastic site.
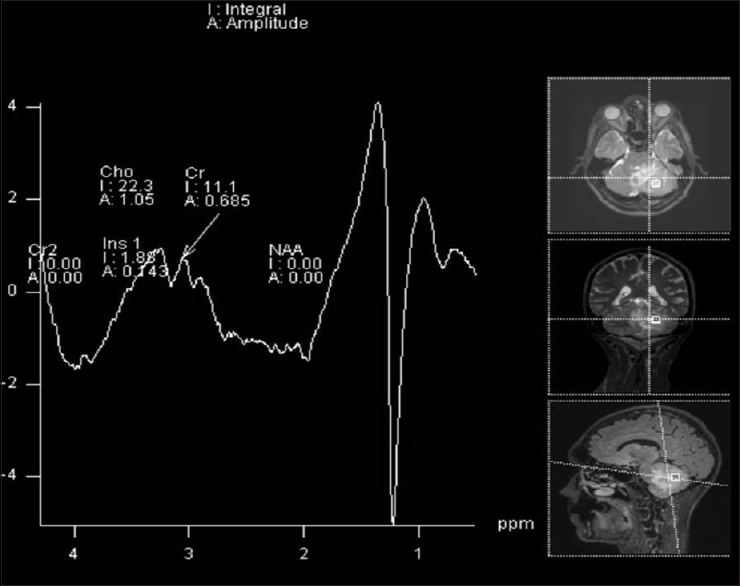
Conclusion:The analysis of the lipid peak by spectroscopy of proton magnetic resonance may indicate the neurological involvement by paracoccidioidomycosis, notably in patients with concomitant risk and pulmonary involvement signals.
Keywords: Central nervous system, computed tomography, magnetic resonance spectroscopy, neuroparacoccidioidomycosis, paracoccidioidomycosis
INTRODUCTION
Paracoccidioidomycosis (PCM) is a systemic mycosis caused by a temperature-dependent dimorphic fungus, the Paracoccidioides brasiliensis. This fungus has significant importance in some countries of Latin America. Humans and some animals, such the armadillo, act as the host. In the natural habitat, the fungus present as the conidia infective form (infective propagules), and once inhaled they give rise to yeast from fungi in the host tissues.[
Males are the most affected, especially rural workers aged 30–50 years. In adults, the most common chronic form is the multifocal one, in which there is a pulmonary manifestation in approximately 90% of the cases. The lungs are considered the primary sites of infection. PCM relates to immunosuppressive conditions, and smoking and alcohol use are associated with this disease.[
CASE REPORT
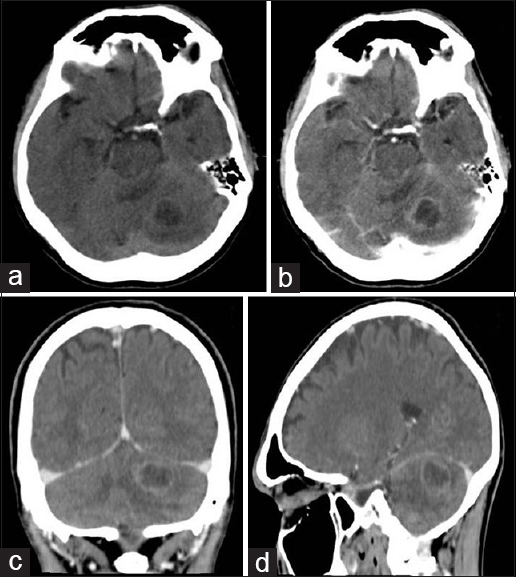
A 49-year-old single man (rural worker), born and raised in Laranjal Paulista-SP, was admitted to our Emergency Room at the Botucatu Medical School University hospital with 3 months history of bilateral occipital headache. Along with a history of active smoking and previous use of alcohol, the patient reported a personal history of mild occipitotemporal injury 3 months ago. On physical examination, left dysdiadochokinesia and three palpable cervical lymph nodes were noted on the left side; one of them with increased dimensions. The patient was submitted to computed tomography (CT) in a 16-row multidetector scanner, which revealed, after iodinated contrast infusion, a nodular hypodense lesion with ring enhancement and associated perilesional edema in the left cerebellar hemisphere [
Figure 2
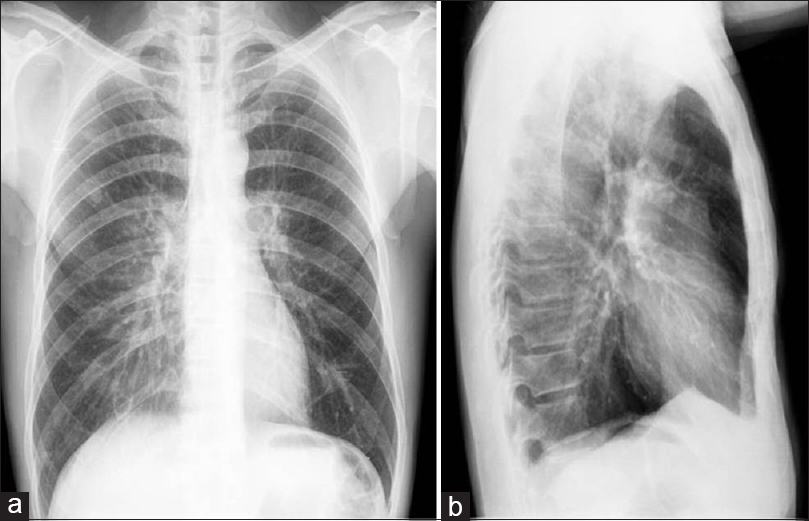
Protocol for high-resolution CT scanning of the chest, axial sections showing lesions with excavated walls, sometimes thin and smooth (a), sometimes thicker and asymmetric (b), and peripheral nodular solid lesions (c and d) in lung parenchyma, as well as area of lowest vascularity and not well defined in both upper and lower regions of the lungs
Figure 3
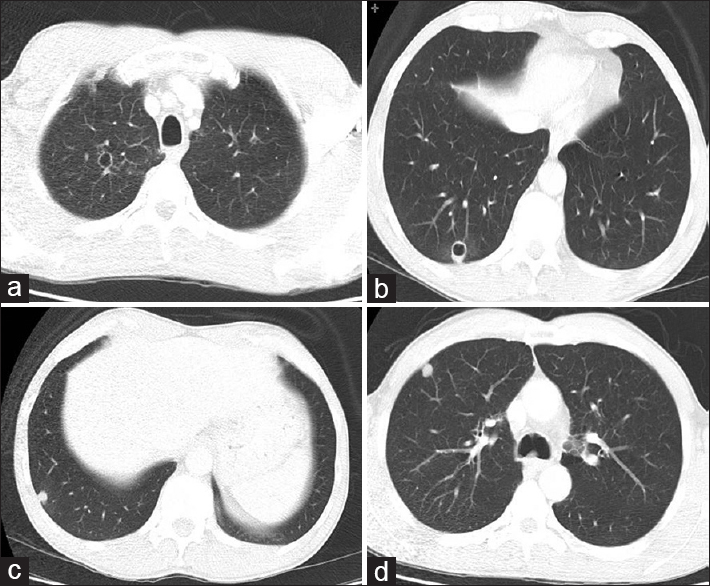
Axial sections of computed tomography before (a) and after (b) the infusion of iodinated contrast agent, revealing nodular hypodense lesion with ring- enhancement, in the left cerebellar hemisphere. Reformation in the coronal (c) and sagittal (d) confirmed the lesion was located in the posterior cranial fossa
Figure 4
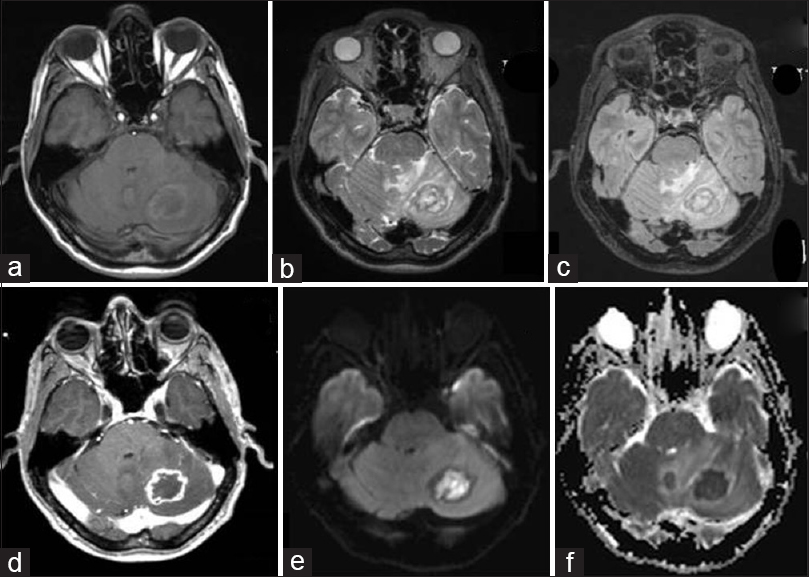
Brain MRI. (a) T1W nodular lesion with peripheral hyperintense rim surrounding a hypointense white matter in the left cerebellar hemisphere. Axial images in (b) T2W sequences and (c) FLAIR-perilesional edema, heterogeneity with peripheral hypointense white matter, center hyperintense. (d) T1W revealed ring-enhancement after intravenous infusion of paramagnetic contrast (gadolinium) in axial section. Axial image of diffusion- DWI sequences (e) lesion hyperintense center; and apparent diffusion coefficient (f), hypointense lesion with restricted diffusion of water molecules
DISCUSSION
A review of national and international literature regarding evaluation of NPCM by neuroimaging methods shows the most common occurrence of NPCM, usually as with multiple granulomas, is the pseudotumoral form. They are commonly seen in CT as supra or infratentorial hypodense injuries, rounded, with variable perilesional edema, ring enhancement on iodinated contrast media. Therefore, CT differential diagnosis include primary or metastatic tumors and pyogenic abscesses.[
CONCLUSION
NPCM is more frequent than it was expected with the advent and the widespread of neuroimaging methods, especially MRI. The patterns for this entity by CT and MRI, in conventional sequences, are considered nonspecific and require differential diagnosis with other lesions such as primary or metastatic tumors and pyogenic abscesses. However, the presence of low signal intensity on the T2-weighted sequence suggests the presence of hemoglobin degradation products.[
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Almeida SM, Queiroz-Telles F, Teive HAG, Ribeiro CEL, Werneck LC. Central nervous system paracoccidioidomycosis: Clinical features and laboratorial findings. J Infect. 2004. 48: 193-8
2. Cunha MLV, Castro CAO, Piekala C, Neto JFA, Pletz ALB. Neuroparacoccidioidomycosis simulating cerebral metastasis: Case report and literature review. J Bras Neurocirurg. 2012. 23: 226-33
3. Elias J, dos Santos AC, Carlotti CG, Colli BO, Canheu A, Matias C. Central nervous system paracoccidioidomycosis: Diagnosis and treatment. Surg Neurol. 2005. 63: 13-21
4. Faria AV, Dabus GC, Zanardi VA, Cendes F. Proton magnetic resonance spectroscopy and magnetic resonance imaging findings in a patient with central nervous system paracoccidioidomycosis. J Neuroimaging. 2004. 14: 377-9
5. Lambertucci JR, Lana-Peixoto MA, Pitella JEH. Paracoccidioidomycosis of the central nervous system. Rev Soc Bras Med Trop. 2001. 34: 395-6
6. Magalhaes AC, Caramelli P, Silva ED, Bacheschi LA, Lo LS, Menezes JR. Magnetic resonance imaging in intracranial paracoccidioidomycosis. J Neuroimaging. 1993. 3: 216-9
7. Paniago AM, de Oliveira PA, Aguiar ES, Aguiar JI, da Cunha RV, Leme LM. Neuroparacoccidioidomycosis: Analysis of 13 cases observed in an endemic area in Brazil. Arq Neuropsiquiatr. 1989. 47: 224-9
8. Pedroso VSP, Vilela MC, Pedroso ERP, Teixeira AL. Paracoccidioidomycosis compromising the central nervous system: a systematic review of the literature. Rev Soc Bras Med Trop. 2009. 42: 691-7
9. Pedroso VSP.editorsExperimental studies and clinical prospects of paracoccidioidomycosis. [Dissertation]. Belo Horizonte: Universidade Federal de. Minas Gerais; 2009. p.
10. Reis F, Collier PP, Souza TF, Lopes GP, Bronzatto E, Silva Junior NA, Pereira RM, Appenzeller S. Neuroparacoccidioidomycosis (NPCM): Magnetic Resonance Imaging (MRI) findings. Mycopathologia. 2013. 175: 181-6
11. Rodacki MA, De Toni G, Borba LA, Oliveira GG. Paracoccidioidomycosis of the central nervous system: CT findings. Neuroradiology. 1995. 37: 636-41
12. Shikanai-Yasuda MA, Telles Filho FQ, Mendes RP, Colombo AL, Moretti ML. Consensus in paracoccidioidomycosis. Rev Soc Bras Med Trop. 2006. 39: 297-310