- Department of Neurosurgery, Saitama Medical Center, Saitama Medical University, Saitama, Japan
Correspondence Address:
Soichi Oya
Department of Neurosurgery, Saitama Medical Center, Saitama Medical University, Saitama, Japan
DOI:10.4103/sni.sni_55_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ryoko Niwa, Soichi Oya, Takumi Nakamura, Taijun Hana, Toru Matsui. Rapid intracranial pressure drop as a cause for posterior reversible encephalopathy syndrome: Two case reports. 05-Jun-2017;8:103
How to cite this URL: Ryoko Niwa, Soichi Oya, Takumi Nakamura, Taijun Hana, Toru Matsui. Rapid intracranial pressure drop as a cause for posterior reversible encephalopathy syndrome: Two case reports. 05-Jun-2017;8:103. Available from: http://surgicalneurologyint.com/surgicalint-articles/rapid-intracranial-pressure-drop-as-a-cause-for-posterior-reversible-encephalopathy-syndrome-two-case-reports/
Abstract
Background:Posterior reversible encephalopathy syndrome (PRES) is characterized by reversible edematous lesions on radiological examinations as well as symptoms of altered consciousness and seizures. To date, the underlying mechanism remains largely unknown.
Case Descriptions:Case 1 is a 72-year-old man with a history of hypertension presented with a subarachnoid hemorrhage. Fourteen days after the successful clipping of a ruptured aneurysm; he experienced inadvertent overdrainage via the intraventricular drain. Nine hours later, he started to have seizures followed by disturbances in consciousness. An emergency magnetic resonance imaging showed multiple high-intensity lesions in the frontal, temporal, parietal, and occipital lobes, basal ganglia, brainstem, and cerebellar hemispheres bilaterally, which are compatible with typical magnetic resonance findings in PRES patients. He was treated conservatively and recovered well. Case 2 is a 68-year-old woman with a mild history of hypertension and a ventriculo-peritoneal shunt for obstructive hydrocephalus, who underwent a cysto-peritoneal shunt placement because of an enlarging symptomatic arachnoid cyst. Immediately following surgery, she experienced disturbances in consciousness and developed status epilepticus. Radiological examinations revealed remarkable shrinkage of the arachnoid cyst and multiple edematous lesions, which led us to strongly suspect PRES. With conservative treatment, her symptoms and the radiological abnormalities disappeared.
Conclusion:Based on the previous literature and our cases, we believe that the association between rapid reduction of intracranial pressure (ICP) and the development of PRES should be recognized because most neurosurgical procedures such as craniotomy or cerebrospinal fluid diversion present a potential risk of rapid reduction of ICP.
Keywords: Cerebrospinal fluid, intracranial pressure, lumbar puncture, posterior reversible encephalopathy syndrome
INTRODUCTION
Posterior reversible encephalopathy syndrome (PRES) was proposed by Hinchey et al. in 1996 to describe characteristic radiological changes indicating brain edema, especially distributed in the posterior circulatory area, which is the most apparent on fluid-attenuated inversion recovery (FLAIR) images.[
As the condition gained recognition, additional characteristics have been reported.[
Here, we present two patients who developed PRES immediately after a rapid reduction in intracranial pressure (ICP). We also reviewed the previous literature and propose that a reduction in ICP may be associated with PRES.
CASE PRESENTATIONS
Case 1
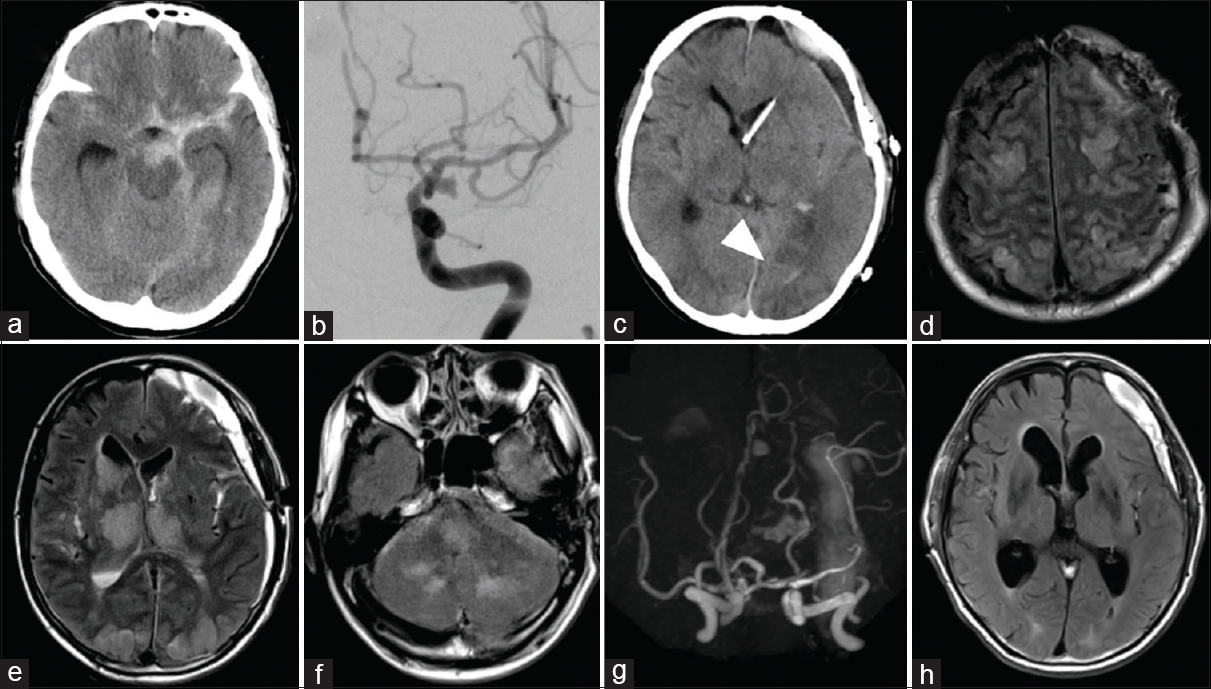
A 72-year-old man on regular medication for hypertension was transported to our hospital complaining of sudden headache followed by disturbed consciousness. Upon arrival, he showed an altered mental status with decreased alertness. He could follow commands but could not speak. He had no other focal deficits, such as hemiparesis or cranial nerve palsy. Computed tomography (CT) showed a subarachnoid hemorrhage (SAH) [
Figure 1
(a) Computed tomography (CT) scan upon admission showing a hematoma in the basal cistern. (b) Anteroposterior view of the left internal carotid angiogram revealing an aneurysm at the bifurcation of the internal carotid artery and the posterior communicating artery. (c) CT on day 14 showing ventricular narrowing and low-density area on the left occipital lobe (arrowhead). (d-f) Magnetic resonance (MR) imaging showed fluid-attenuated inversion recovery (FLAIR) images depicting diffuse high-intensity lesions in the bilateral frontal, temporal, parietal, and occipital lobes, and basal ganglia, brainstem, and cerebellum. (g) MR angiography showing no significant vasospasm. (h) MR imaging obtained on day 30 showing a complete resolution of high-intensity lesions.
Case 2
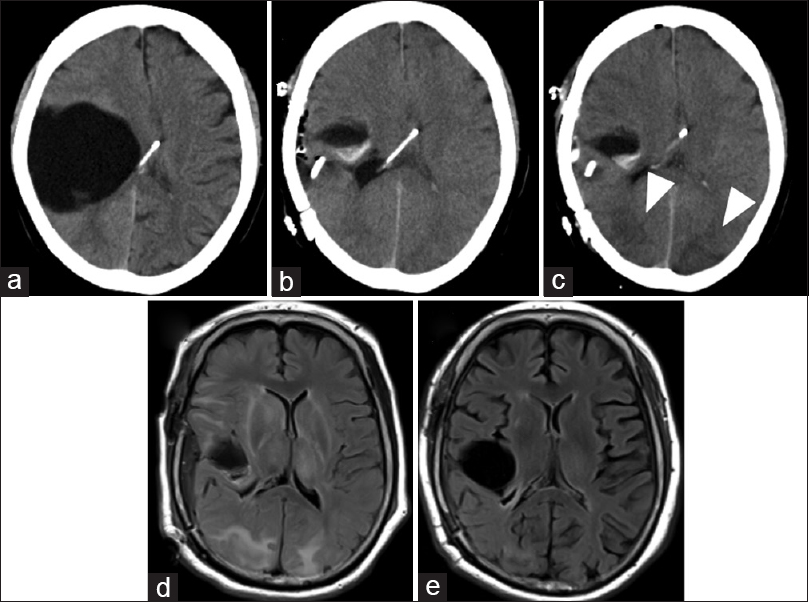
A 68-year-old woman presented at our outpatient clinic complaining of left hemiparesis. She had a history of well-controlled hypertension and had a VP shunt placed 18 years ago for obstructive hydrocephalus due to midbrain cavernous malformation. CT images showed an enlargement of the right frontotemporal arachnoid cyst [
Figure 2
(a) CT scan demonstrating a large arachnoid cyst on the right frontotemporal region. (b) Immediate postoperative CT revealing a remarkable shrinkage of the cyst. (c) CT obtained 1 day after surgery showing low-density areas in the bilateral occipital lobes. (d) MR FLAIR image on the fourth day after surgery demonstrating diffuse high-intensity areas in the temporal, parietal, and occipital lobes on both sides, and in the right frontal lobe. (e) MR FLAIR image 27 days after surgery showing an almost complete disappearance of lesions
DISCUSSION
Despite its name containing the term “posterior,” PRES is believed to occur in the posterior circulatory areas as well as in the temporal lobes or frontal lobes,[
Although ICP was not directly measured, a rapid reduction in ICP appeared to occur in both of our cases. In Case 1, radiological clinical signs of PRES presented approximately 6 h after the inadvertent massive CSF drainage, indicating that the rapid decrease in ICP could have been the main trigger of PRES. Similarly, the postoperative CT in Case 2 showed substantial shrinkage of the cyst, suggesting a possible abrupt event that decreased ICP during surgery. Ho et al. proposed that reduction in ICP decreases ventricular size, resulting in mechanical stress to vessels and causing vasoconstriction and vasogenic edema.[
Hammad et al. recently reported a case of PRES secondary to CSF leak and intracranial hypotension, and also reviewed 10 cases of PRES that developed after spinal or epidural tap.[
In Case 2, the cysto-peritoneal shunt for the large arachnoid cyst might have caused an acute drop in ICP that resulted in PRES, a novel situation that has not been reported in the literature, to the best of our knowledge. Theoretically, several neurosurgical procedures are at high risk for unintentional hyperperfusions. Although rare, surgeons should be aware of the possibility of PRES in patients with severe headache, seizure, and disturbances in consciousness that cannot be explained by other medical conditions.
The prognosis of PRES is generally good and almost all patients make full recoveries or return to a normal life with minor deficits.[
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Amin-Hanjani S, Schwartz RB, Sathi S, Stieg PE. Hypertensive encephalopathy as a complication of hyperdynamic therapy for vasospasm: Report of two cases. Neurosurgery. 1999. 44: 1113-6
2. Avecillas-Chasín JM, Gómez G, Jorquera M, Alvarado LR, Barcia JA. Delayed posterior reversible encephalopathy syndrome (PRES) after posterior fossa surgery. Acta Neurochir. 2013. 155: 1045-7
3. Awori J, Rajajee V, Gemmete JJ, Chaudhary N, Thompson BG, Pandey AS. Posterior reversible encephalopathy syndrome following hemodynamic treatment of aneurysmal subarachnoid hemorrhage-induced vasospasm. J Clin Neurosci. 2016. 26: 33-6
4. Datar S, Singh TD, Fugate JE, Mandrekar J, Rabinstein AA, Hocker S. Albuminocytologic Dissociation in Posterior Reversible Encephalopathy Syndrome. Mayo Clin Proc. 2015. 90: 1366-71
5. Dhar R, Dacey R, Human T, Zipfel G. Unilateral posterior reversible encephalopathy syndrome with hypertensive therapy of contralateral vasospasm: Case report. Neurosurgery. 2011. 69: E1176-81
6. Doherty H, Hameed S, Ahmed I, Russell IF. Post-dural puncture headache and posterior reversible encephalopathy syndrome: A misdiagnosis or co-presentation. Int J Obstet Anesth. 2014. 23: 279-82
7. Eran A, Barak M. Posterior reversible encephalopathy syndrome after combined general and spinal anesthesia with intrathecal morphine. Anesth Analg. 2009. 108: 609-12
8. Fok A, Chandra RV, Gutman M, Ligtermoet M, Seneviratne U, Kempster P. Posterior Reversible Encephalopathy Syndrome and Subarachnoid Hemorrhage After Lumboperitoneal Shunt for Fulminant Idiopathic Intracranial Hypertension. J Neuroophthalmol. 2016. 36: 164-6
9. Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA. Posterior reversible encephalopathy syndrome: Associated clinical and radiologic findings. Mayo Clin Proc. 2010. 85: 427-32
10. Giraldo EA, Fugate JE, Rabinstein AA, Lanzino G, Wijdicks EFM. Posterior reversible encephalopathy syndrome associated with hemodynamic augmentation in aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2011. 14: 427-32
11. González Quarante LH, Mena-Bernal JH, Martín BP, Ramírez Carrasco M, Muñoz Casado MJ, Martínez de Aragón A. Posterior reversible encephalopathy syndrome (PRES): A rare condition after resection of posterior fossa tumors: Two new cases and review of the literature. Childs Nerv Syst. 2016. 32: 857-63
12. Grelat M, Debaux JB, Sautreaux JL. Posterior reversible encephalopathy syndrome after depletive lumbar puncture: A case report. J Med Case Rep. 2014. 8: 261-
13. Hammad T, DeDent A, Algahtani R, Alastal Y, Elmer L, Medhkour A. Posterior Reversible Encephalopathy Syndrome Secondary to CSF Leak and Intracranial Hypotension: A Case Report and Literature Review. Case Rep Neurol Med. 2015. p. 538523-5
14. Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996. 334: 494-500
15. Ho CM, Chan KH. Posterior reversible encephalopathy syndrome with vasospasm in a postpartum woman after postdural puncture headache following spinal anesthesia. Anesth Analg. 2007. 105: 770-2
16. Hong JY, Jee YS, Lee IH, Shin JS, Choi HJ. Posterior reversible encephalopathy syndrome after cesarean section under spinal anesthesia. Korean J Anesthesiol. 2007. 52: S86-90
17. Horie N, Morikawa M, Kitagawa N, Nagata I. Cerebellar variant of posterior reversible encephalopathy syndrome (PRES) after coil embolization for the hemorrhagic dissecting aneurysm. Acta Neurochir. 2011. 153: 1143-4
18. Jang HW, Lee HJ. Posterior reversible leukoencephalopathy due to “triple H” therapy. J Clin Neurosci. 2010. 17: 1059-61
19. Kuroda H, Kashimura H, Murakami T, Endo H, Mase T, Ogasawara K. Early onset of PRES in a patient with a subarachnoid haemorrhage due to a ruptured intracranial aneurysm. Br J Neurosurg. 2014. 28: 785-6
20. Liman TG, Bohner G, Heuschmann PU, Endres M, Siebert E. The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: The retrospective Berlin PRES study. J Neurol. 2012. 259: 155-64
21. Madaelil TP, Dhar R. Posterior reversible encephalopathy syndrome with thalamic involvement during vasopressor treatment of vertebrobasilar vasospasm after subarachnoid hemorrhage. BMJ Case Rep 2015. 2015. p.
22. Minai FN, Hasan SF, Sheerani M. Post-dural puncture posterior reversible encephalopathy syndrome. J Coll Physicians Surg Pak. 2011. 21: 37-9
23. Moriarity JL, Lim M, Storm PB, Beauchamp NJ, Olivi A. Reversible posterior leukoencephalopathy occurring during resection of a posterior fossa tumor: Case report and review of the literature. Neurosurgery. 2001. 49: 1237-9
24. Muhammad S, Güresir Ã, Greschus S, Scorzin J, Vatter H, Güresir E. Posterior Reversible Encephalopathy Syndrome as an Overlooked Complication of Induced Hypertension for Cerebral Vasospasm: Systematic Review and Illustrative Case. Stroke. 2016. 47: 519-22
25. Orehek EK, Burns JD, Koyfman F, Azocar RJ, Holsapple JW, Green DM. Postpartum trifecta: Simultaneous eclamptic intracerebral hemorrhage, PRES, and herniation due to intracranial hypotension. Neurocrit Care. 2012. 17: 434-8
26. Patel AJ, Fox BD, Fulkerson DH, Yallampalli S, Illner A, Whitehead WE. Posterior reversible encephalopathy syndrome during posterior fossa tumor resection in a child. J Neurosurg Pediatr. 2010. 6: 377-80
27. Pradhan A, Jairam A, Kumar RSV, Srivastava A, Sreevastava D, Dutta A. Posterior reversible encephalopathy syndrome posttransplantation: A case report of possible association with cerebrospinal fluid leak after epidural catheterization. Transplant Proc. 2009. 41: 1957-60
28. Prout RE, Tuckey JP, Giffen NJ. Reversible posterior leucoencephalopathy syndrome in a peripartum patient. Int J Obstet Anesth. 2007. 16: 74-6
29. Pugliese S, Finocchi V, Borgia ML, Nania C, Vella Della B, Pierallini A. Intracranial hypotension and PRES: Case report. J Headache Pain. 2010. 11: 437-40
30. Sanelli PC, Jacobs MA, Ougorets I, Mifsud MJ. Posterior reversible encephalopathy syndrome on computed tomography perfusion in a patient on “Triple H” therapy. Neurocrit Care. 2005. 3: 46-50
31. Shah R, Kubisz-Pudelko A, Reid J. Posterior reversible encephalopathy syndrome following an inadvertent dural puncture during an emergency laparotomy for ischemic colitis-a case report. Local Reg Anesth. 2014. 7: 1-4
32. Shields LBE, Johnson JR, Shields CB. Posterior reversible encephalopathy syndrome following a thoracic discectomy-induced dural leak: Case report. J Neurosurg Spine. 2016. 25: 586-90
33. Sorour M, Sayama C, Couldwell WT. Posterior Reversible Encephalopathy Syndrome after Surgical Resection of a Giant Vestibular Schwannoma: Case Report and Literature Review. J Neurol Surg A Cent Eur Neurosurg. 2016. 77: 274-9
34. Torrillo TM, Bronster DJ, Beilin Y. Delayed diagnosis of posterior reversible encephalopathy syndrome (PRES) in a parturient with preeclampsia after inadvertent dural puncture. Int J Obstet Anesth. 2007. 16: 171-4
35. Voetsch B, Tarlov N, Nguyen TN, DeFusco C, Barest GD, Norbash A. Asymmetric posterior reversible encephalopathy syndrome complicating hemodynamic augmentation for subarachnoid hemorrhage-associated cerebral vasospasm. Neurocrit Care. 2011. 15: 542-6
36. Wartenberg KE, Parra A. CT and CT-perfusion findings of reversible leukoencephalopathy during triple-H therapy for symptomatic subarachnoid hemorrhage-related vasospasm. J Neuroimaging. 2006. 16: 170-5