- Department of Neurosurgery, Faculty of Medicine, Universitas Padjadjaran–Dr. Hasan Sadikin Hospital, Bandung, West Java, Indonesia
Correspondence Address:
Ahmad Faried
Department of Neurosurgery, Faculty of Medicine, Universitas Padjadjaran–Dr. Hasan Sadikin Hospital, Bandung, West Java, Indonesia
DOI:10.4103/sni.sni_26_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ahmad Faried, Wargian Hadisaputra, Muhammad Z. Arifin. Recurrence case of rare scalp dermatofibrosarcoma protuberans: Two case reports of a wide radical excision, craniectomy bone involvement followed by cranioplasty and reconstruction. 26-May-2017;8:82
How to cite this URL: Ahmad Faried, Wargian Hadisaputra, Muhammad Z. Arifin. Recurrence case of rare scalp dermatofibrosarcoma protuberans: Two case reports of a wide radical excision, craniectomy bone involvement followed by cranioplasty and reconstruction. 26-May-2017;8:82. Available from: http://surgicalneurologyint.com/surgicalint-articles/recurrence-case-of-rare-scalp-dermatofibrosarcoma-protuberans-two-case-reports-of-a-wide-radical-excision-craniectomy-bone-involvement-followed-by-cranioplasty-and-reconstruction/
Abstract
Background:Dermatofibrosarcoma protuberans (DFSP) is a rare low-grade sarcoma of the fibroblast originating from the dermal layer of the skin, characterized by a locally aggressive growth and high rate of local recurrence.
Case Description:Two patients underwent a wide radical excision of recurrent scalp DFSP which was reconstructed with translational skin flap and split-thickness skin graft. We described above cases several years ago with a local excision of the tumor; recently, they developed local recurrence of DFSP with calvarial involvement. We then performed a wide radical excision, with craniectomy of the cranial defect followed by cranioplasty using titanium mesh, continuing with reconstruction.
Conclusion:A successful treatment and management depends on achieving local control and preventing cosmetic and functional deficit; all efforts should be made for complete excision. Postoperative follow-up recommended for highly suspicious cases and annual checkups should be performed up to 5 years after definitive therapy.
Keywords: A wide radical excision, craniectomy and cranioplasty, recurrence of dermatofibrosarcoma protuberans, translational flap and split-thickness skin graft
INTRODUCTION
Dermatofibrosarcoma protuberans (DFSP) is a neoplasm originating from the fibroblast with characteristic aggressive mesenchymal tumor; its local recurrence rate is up to 60%.[
This article reported our recurrent case of scalp DFSP, reviews this locally aggressive growth tumor with high rates of local recurrence, surgical technique, and its management, as well as with follow-up recommendations for this unusual neoplasm.
CASE REPORTS
Case 1
We present a case of a 29-year-old adult male whose main complaint was a recurrent lump, a painless lesion, on his mid frontal region after tumor excision who was diagnosed with DFSP 4 years ago [Figure
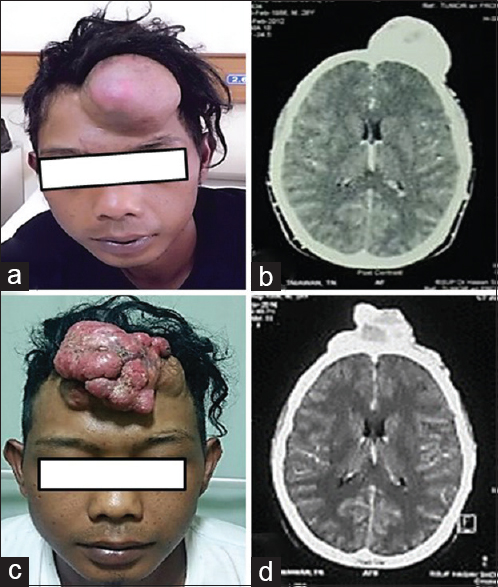
Figure 1
(a) Lump in mid frontal region in male patient (before excision in 2012), mass size 12 × 10 × 6 cm. (b) Head CT scan showing soft tissue attenuation irregular mass single lesion lobulated in mid frontal region. (c) Lump in mid frontal region in same patient after operated 4 years ago with mass size 12 × 10 × 5 cm. (d) Head CT scan showing soft tissue attenuation irregular mass multiple lesion lobulated in mid frontal
Under general anesthesia, the patient was positioned supine with his back elevated approximately 20 degrees. A wide radical excision with 4 cm distance from neoplasm margin and translational skin flap line was drawn [Figure
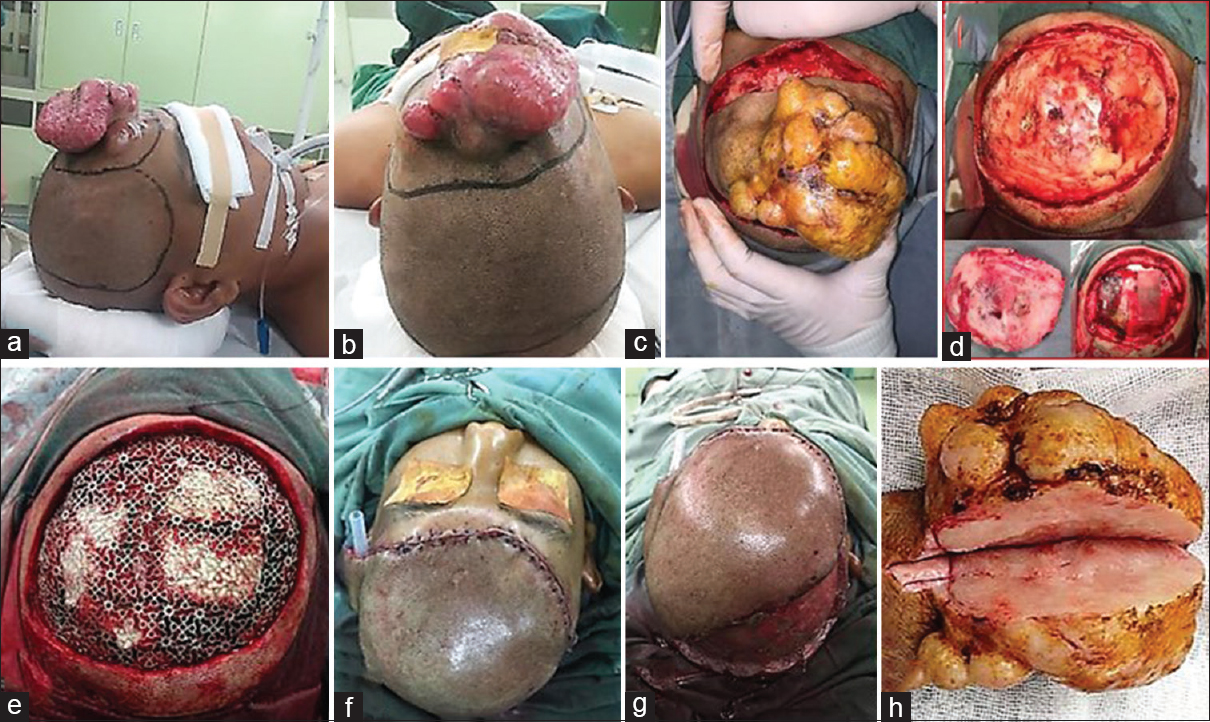
Figure 2
(a-c) A wide radical excision with 4 cm distance from neoplasm margin and translational skin flap line was drawn. (d) After wide excision was performed, calvarial defect was observed without involvement of duramater below; as frontal bone craniectomy was performed. (e) A bone defect 10 × 8cm was made and cranioplasty using titanium mesh was placed. (f) The skin defect was then reconstructed by translational skin flap and (g) split-thickness skin graft. (h) Mass with an irregular, skin colored, multiple lobulated mass, 12 × 10 × 5 cm in diameter was excised
As in histopathological findings, using hematoxylin and eosin (HE) stained sections showed a densely cellular and poorly circumscribed tumor in the dermis layer, comprising interwoven bundles and fascicles of uniform spindle shaped cells arranged in a “storiform” or “cartwheel” pattern [
Case 2
A 49-year-old male presented with the main complaint of a recurrent lump, a painless lesion, on his mid parietooccipital region after tumor excision; the patient was diagnosed with DFSP 3 years ago. History revealed that the recurrence of DFSP occurred in the second year after the first tumor excision. On physical examination, there was an irregular, skin-colored, multiple lobulated mass, measuring 8 × 6 × 4 cm in diameter, with a fixated base; head CT showed no intracranial mass [
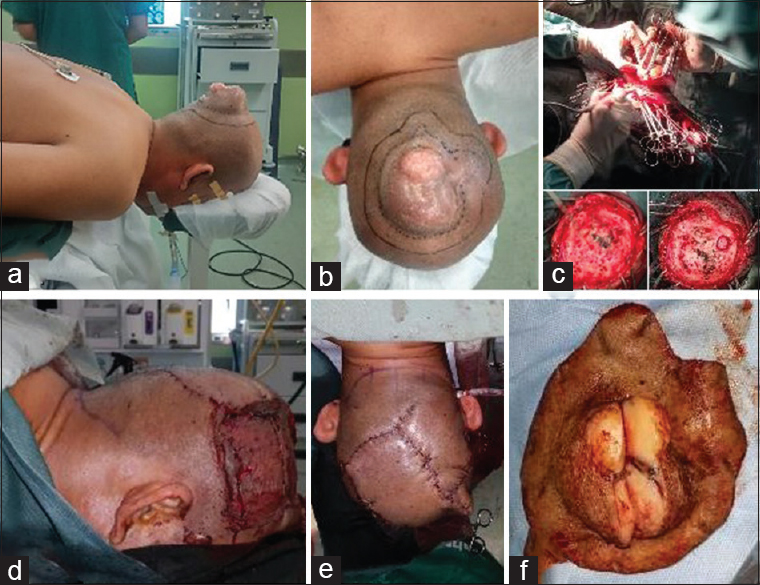
Figure 3
(a, b) A wide radical excision with 4 cm distance from neoplasm margin and translational skin flap line was drawn. (c) After wide excision were performed, calvarial defect was observed without involvement of duramater below; as frontal bone craniectomy was performed. (d, e) The skin defect was then reconstructed by translational skin flap and (f). Mass with an irregular, skin colored, multiple lobulated mass, 8 × 6 × 4 cm in diameter was excised
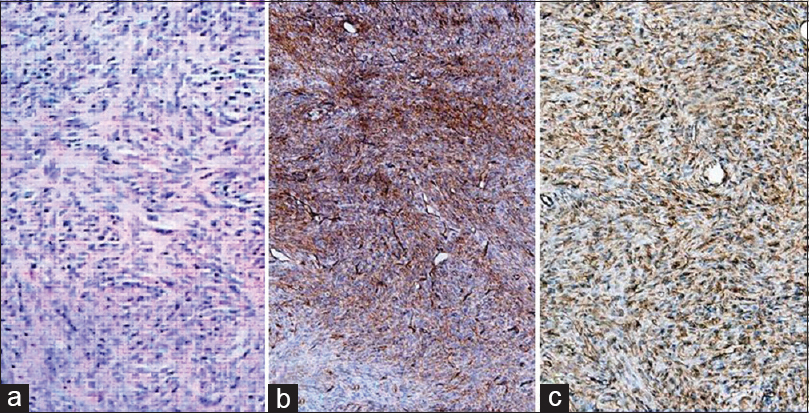
Figure 4
(a) Section showing spindle cells arranged in short fascicles, storiform pattern, oval nuclei, vesicular chromatin, inconspicuous nucleoli and scanty to moderate cytoplasm (HE, 100×). (b) Section showing tumor cell diffuse strong positive staining for CD-34 (100×). (c) Section showing tumor cell diffuse strong positive staining for vimentin (100×)
Under general anesthesia, the patient was positioned. A wide radical excision with 4 cm distance from neoplasm margin and translational skin flap line was drawn [
DISCUSSION
The management of scalp DFSP, immunohistochemical demonstration of CD34, and vimentin are important features for diagnosing DFSP; in which both proteins showed strong positivity were noted in our cases. Recurrent lesions, masses measuring more than 5 cm, and those with calvarial involvement need team effort from both neurosurgeon and plastic surgeon for reconstruction. One study observed recurrence rates after wide local excision performed during 2000–2012.[
Scalp reconstruction was then performed using translational local skin flap and split-thickness skin grafts. Local skin flaps in our cases are the most suited for reconstruction because they have similar characteristic feature with the defect area. Other options are free flap reconstruction that require technical expertise, increased operative time, and intensive postoperative care; most of free tissue transfers commonly resulted in hairless reconstructive area. Wide excision followed by cranioplasty and flap reconstruction provide the best possible esthetic and functional results.
Clinically, DFSP initially presents typically superficial in location as a cutaneous pink to red-bluish painless plaque which grows more nodular-exophytic growth pattern with time. Histopathologically, DFSP consisted densely packed, monomorphous, clump of spindle-shape cells arrange “storiform” or “cartwheel” of uniform appearing fibroblast; whereas at the periphery there was diffuse infiltrative into subcutis. IHC staining demonstrate spindle cells with strong positivity for CD-34 and vimentin expression as an important feature for diagnosing DFSP. CD-34 positivity is particularly useful for the differential diagnosis of DFSP from the fibrohistiocytics lesions that shown negative CD-34 expression. The fibrosarcomatous variant of DFSP (FS-DFSP), loss of CD-34 gradually less than 50%, represents a form of tumor progression and associated with a significantly more aggressive clinical course than ordinary DFSP.[
CONCLUSION
A successful treatment and management depends on the achievement of local control and the prevention of cosmetic and functional deficit; all efforts should be made for complete excision with negative margin. Postoperative follow-up using magnetic resonance imaging or positron emission tomography is recommended for highly suspicious cases, and annual checkups should performed up to 5 years after definitive therapy.
CONSENT
Informed consent was obtained from both patients for publication of this two case reports and any accompanying images. Their family was present at the time.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
All authors had examined, treated, observed and followed up the patient and participated in writing the manuscript. All authors has read and approved of the final manuscript.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Akram J, Wooler G, Lock-Andersen J. Dermatofibrosarcoma protuberans: Clinical series, national Danish incidence data and suggested guidelines. J Plast Surg Hand Surg. 2014. 48: 67-73
2. Arifin MZ, Yudoyono F, Dahlan RH, Hernowo BS, Sutiono AB, Faried A. A Rare Scalp Dermato-fibrosarcoma Protuberans. Surg Neurol Int. 2014. 5: 45-
3. Bhatnagar A, Srivastava A, Sahu RN. Management of Recurrent Dermatofibro Sarcoma Protuberance of Scalp-A Reconstructive Challenge. Indian J Surg Oncol. 2013. 4: 15-8
4. Kim BJ, Kim H, Jin US, Minn KW, Chang H. Wide local excision for dermatofibrosarcoma protuberans: A single-center series of 90 patients. Biomed Res Int 2015. 2015. p. 642549-
5. Mentzel T, Beham A, Katenkamp D, Dei Tos AP, Fletcher CD. Fibrosarcomatous (“high-grade”) dermatofibrosarcoma protuberans: Clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance. Am J Surg Pathol. 1998. 22: 576-87
6. Miller SJ, Alam M, Andersen JS, Berg D, Bichakjian CK, Bowen GM. Dermatofibrosarcoma protuberans. J Natl Compr Canc Netw. 2012. 10: 312-8
7. Pallure V, Dupin N, Guillot B. Surgical treatment of Darier-Ferr and dermatofibrosarcoma: A systematic review. Dermatol Surg. 2013. 39: 1417-33