- Department of Neurosurgery, Loma Linda University School of Medicine, Loma Linda,
- Department of Basic Science, School of Medicine, Loma Linda University, Loma Linda,
- Department of General Surgery, Tripler Army Medical Center, Honolulu, Hawaii, United States.
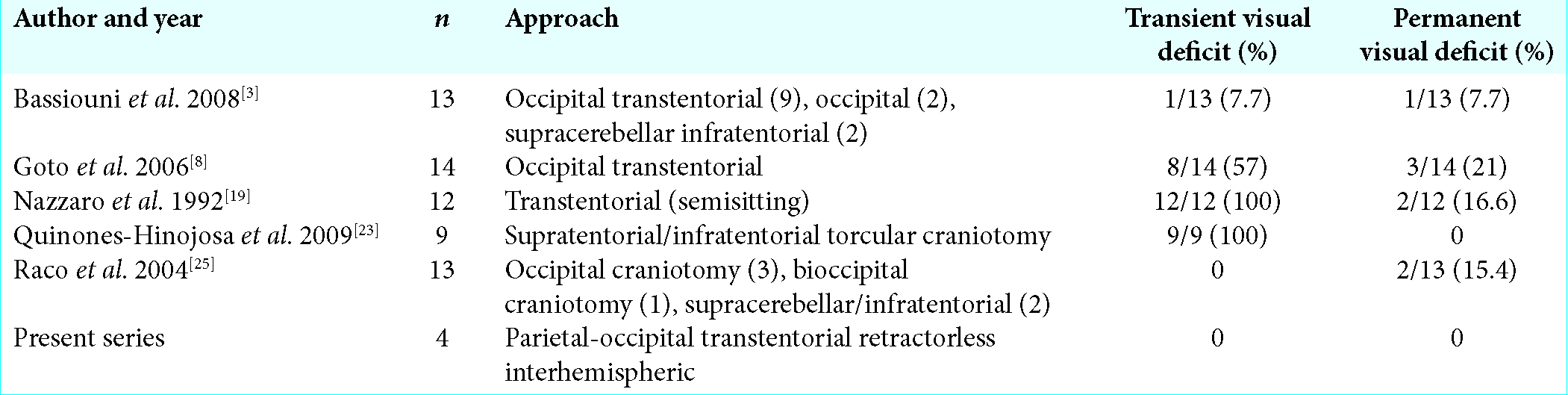
- Department of Neurosurgery, Loma Linda University, Loma Linda,
- Department of Basic Sciences, Loma Linda University Medical Center, Loma Linda,
- Department of Neurosciences, University of Southern California, Los Angeles, California, United States.
- Center for Neurosciences Research, Loma Linda University, Loma Linda,
Correspondence Address:
Miguel Angel Lopez-Gonzalez
Center for Neurosciences Research, Loma Linda University, Loma Linda,
DOI:10.25259/SNI-117-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Miguel Angel Lopez-Gonzalez, Andrew Jaeger, Brett Kaplan, Timothy Marc Eastin, Lydia Kore, Vadim Gospodarev, Puja D. Patel, Fransua Sharafeddin. Retractorless interhemispheric transtentorial approach for large lesions in the posterior incisural space. 28-Jun-2019;10:130
How to cite this URL: Miguel Angel Lopez-Gonzalez, Andrew Jaeger, Brett Kaplan, Timothy Marc Eastin, Lydia Kore, Vadim Gospodarev, Puja D. Patel, Fransua Sharafeddin. Retractorless interhemispheric transtentorial approach for large lesions in the posterior incisural space. 28-Jun-2019;10:130. Available from: http://surgicalneurologyint.com/surgicalint-articles/9440/
Abstract
Background: Surgical resection of lesions in the posterior incisural space presents a significant surgical challenge, which may result in postoperative visual complications and other neurological deficits. We, therefore, describe a retractorless interhemispheric transtentorial approach that avoids surrounding brain structures with positive outcomes and no complications or visual damage.
Case Description: We present four cases
Conclusion: A carefully executed retractorless interhemispheric approach in select cases is an effective option to reduce morbidity and prevent visual complications when removing lesions in the posterior tentorial incisure.
Keywords: Falcotentorial, Hemangioblastoma, Interhemispheric, Meningioma, Retractorless
INTRODUCTION
The tentorial incisura is the anterior opening between the free edge of the tentorium cerebelli and the petroclival ridge for the passage of the brainstem where the supratentorial space communicates with the infratentorial space. Within it, the posterior incisural space is a complex region located posterior to the midbrain where the pineal gland and the tentorial notch are located. The posterior incisural space is home to significant neurovascular structures such as the Vein of Galen, basal vein of Rosenthal, internal occipital vein, posterior cerebral artery, splenium of corpus callosum, superior vermis, and trochlear nerve.[
CASE REPORTS
Patients were evaluated and managed at our institution from November 2016 to November 2018.
Patient 1
A 62-year-old female with a history of hypertension, hyperlipidemia, and cerebrovascular accident was followed at another institution for several years with a gradually enlarging falcotentorial lesion. The patient presented to our emergency room with intensifying occipital headaches that were throbbing and pressurized in nature along with worsening balance and gait difficulties. On physical exam, the patient had residual stroke deficits with left upper extremity weakness, right central face palsy, left dysmetria, and worsening gait imbalance. Preoperative imaging showed a 3.6 cm × 3.5 cm × 3 cm enhancing lesion in the posterior incisural space with severe brainstem compression [
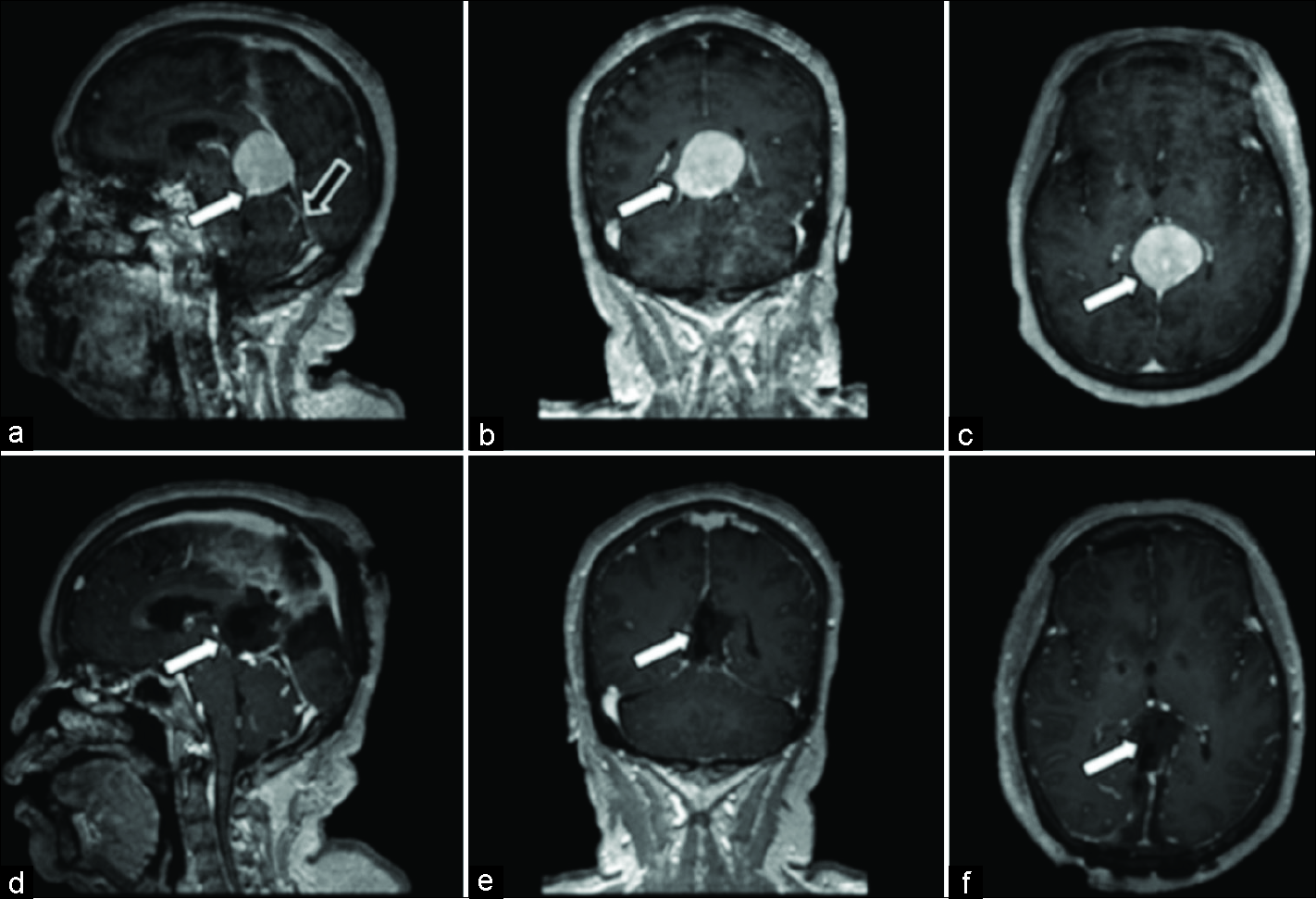
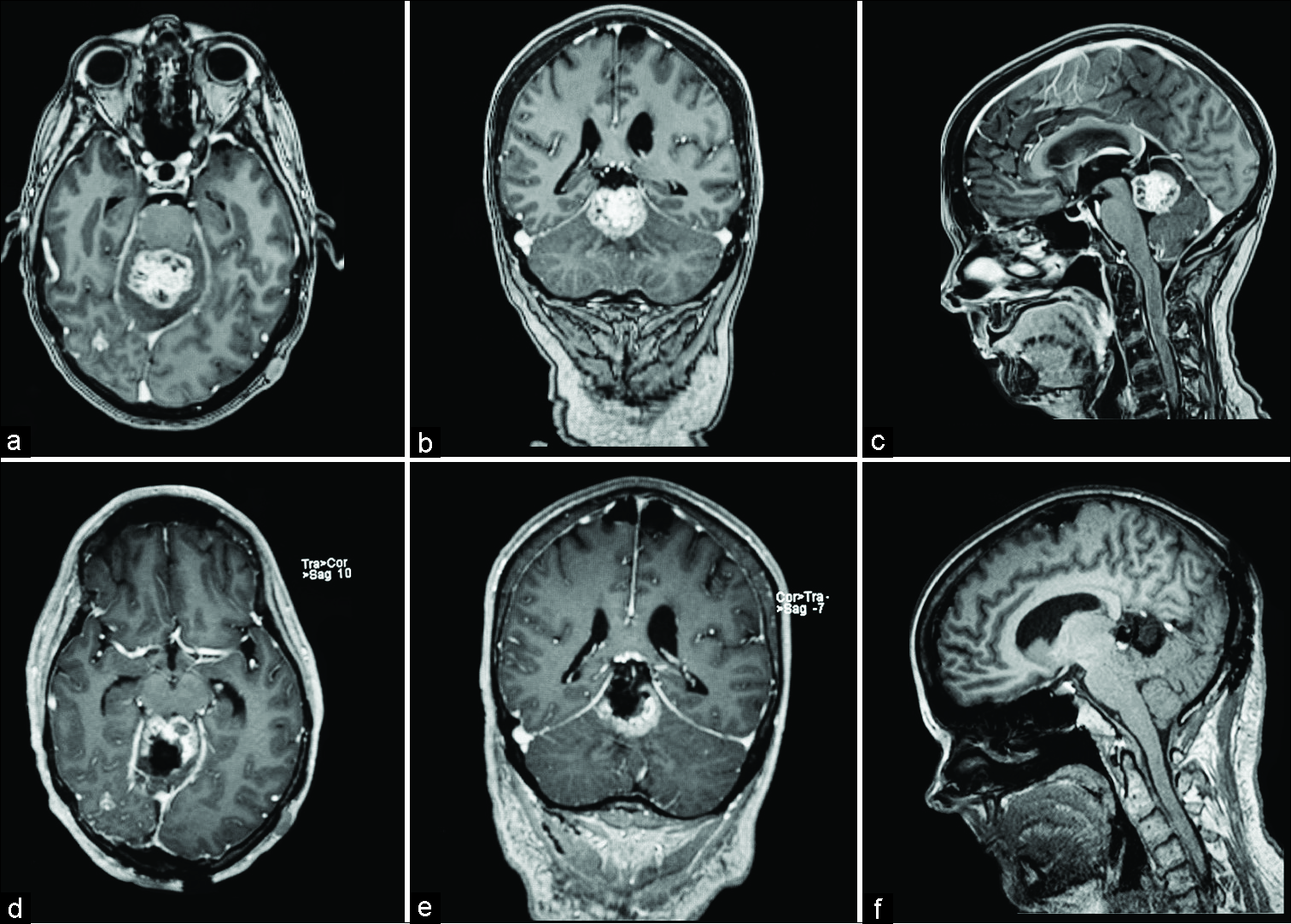
Figure 1:
Magnetic resonance images for patient 1. (a) Preoperative postcontrast T1 sagittal, avidly enhancing mass measuring 3.6 cm anterior-posterior (white arrow) along the tentorium, compressing the aqueduct of Sylvius. Tentorial angle is excessively steep (black arrow), which prohibits an infratentorial approach. (b) Preoperative postcontrast T1 coronal, redemonstration of hyperintense mass measuring 3 cm transverse (white arrow). (c) Preoperative postcontrast T1 axial, hyperintense mass measuring 3.5 cm cranial-caudal (white arrow). (d) Postoperative postcontrast T1 sagittal, postsurgical changes demonstrating gross total resection of pineal region mass (white arrow). (e) Postoperative postcontrast T1 coronal, postsurgical changes demonstrating gross total resection of pineal region mass (white arrow). (f) Postoperative postcontrast T1 axial, postsurgical changes demonstrating gross total resection of pineal region mass (white arrow).
The patient underwent two stages of resection: an initial left parietal-occipital transtentorial retractorless interhemispheric approach under general anesthesia with electrophysiological monitoring followed 2 days later by a right sided approach for complete tumor resection [
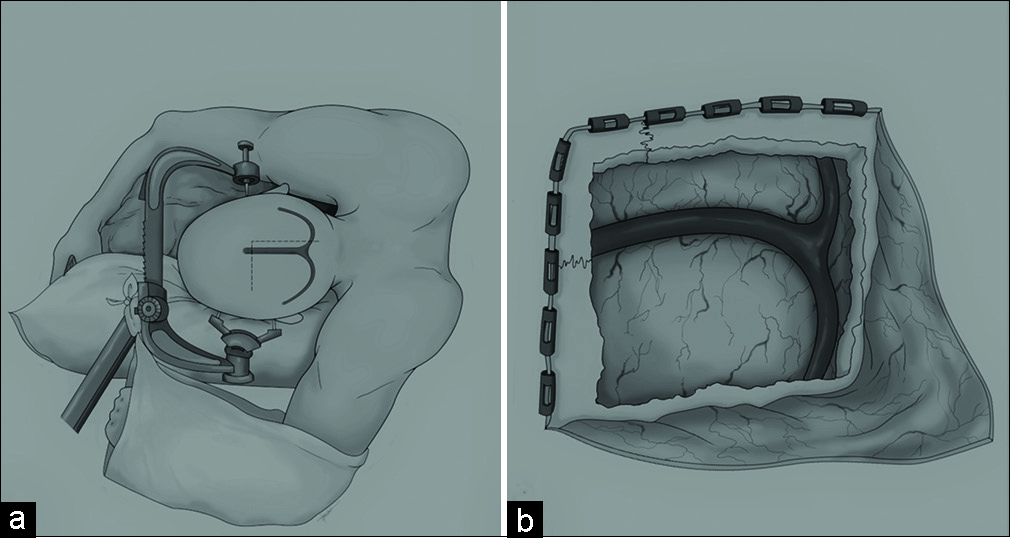
For stage one, the patient was placed in a lateral position with their left side facing down and the sagittal plane parallel to the floor. Gravity served to retract the parietal-occipital lobe [
Complete resection of the tumor, including dural attachments, was achieved with no surgical complications. The ventriculostomy was removed on postoperative day 2. The patient continued to experience her baseline remote stroke sequelae symptoms, although her gait imbalance deficit improved in approximately 2 months. Postoperatively and at 6-month follow-up, radiographic imaging showed no residual lesion. Pathological examination confirmed a WHO Grade I fibrous meningioma.
Patient 2
A 38-year-old female with no significant medical history presented with severe headaches and nausea that was exacerbated by activities such as bending over and lifting. The patient was previously seen by other neurosurgeons outside of the United States and had undergone an attempted biopsy for a pineal region lesion through a frontal endoscopic approach. This patient developed Parinaud syndrome, diplopia, and gait imbalance which lasted approximately 1 month. Later, they had a second stereotactic biopsy that revealed a WHO Grade 1 meningothelial/fibroblastic meningioma. The patient was neurologically intact by the time of our initial evaluation. Imaging revealed a 3.5 cm × 3.1 cm × 3 cm large pineal region enhancing lesion with brainstem (midbrain in the tectal plate) and cerebellar compression [
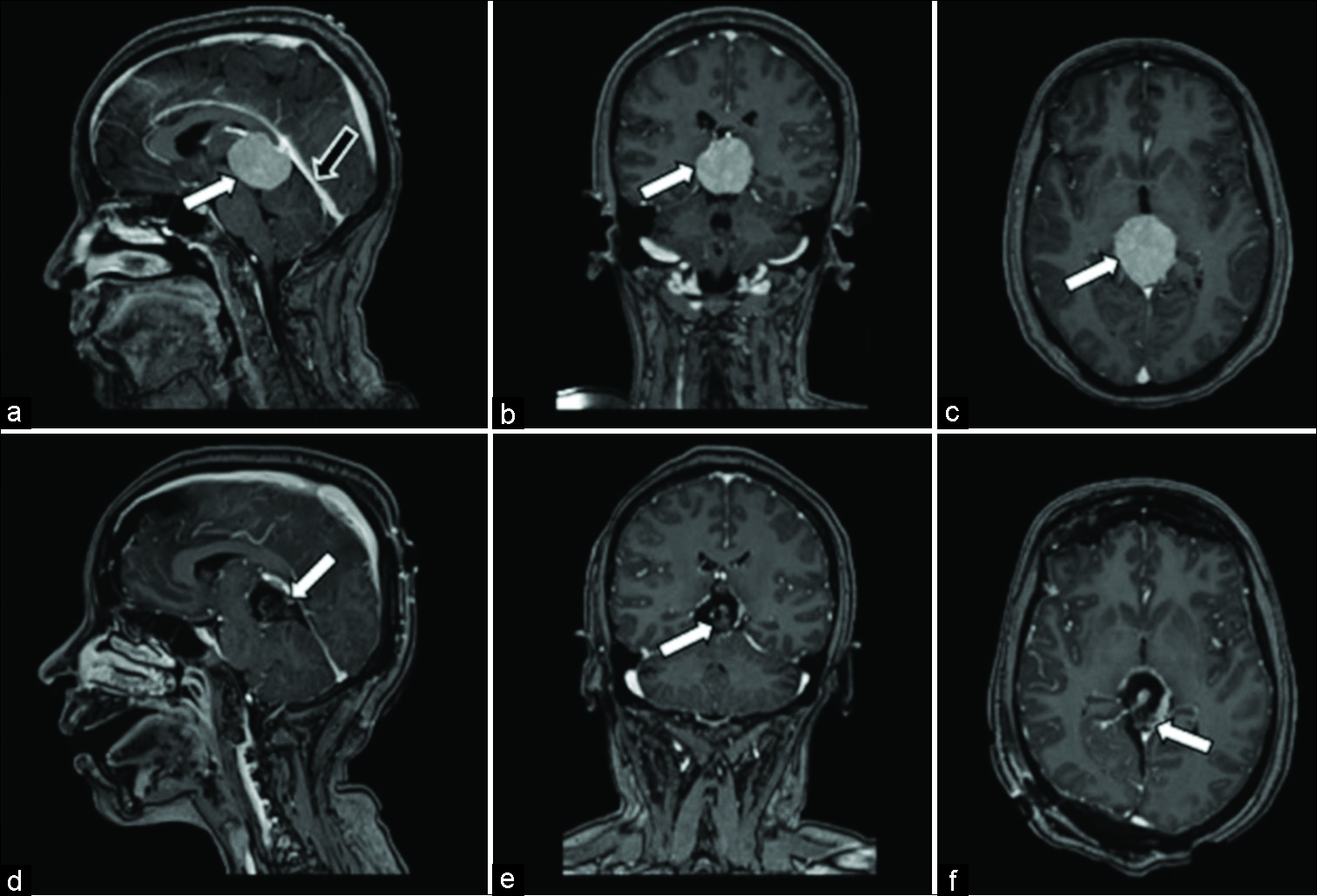
Figure 3:
Magnetic resonance Images for patient 2. (a) Preoperative postcontrast T1 sagittal, homogeneously enhancing ovoid mass centered in the quadrigeminal cistern/pineal region which measures 3.5 cm anterior-posterior (white arrow), compressing the cerebral aqueduct. Tentorial angle is excessively steep (black arrow), which prohibits an infratentorial approach. (b) Preoperative postcontrast T1 coronal, homogeneously enhancing mass measuring 3.1 cm transverse (white arrow). (c) Preoperative postcontrast T1 axial, homogeneously enhancing mass measuring 3 cm cranial-caudal (white arrow). (d) Postoperative postcontrast T1 sagittal, postsurgical changes demonstrating residual tumor capsule (white arrow) attached to segments of the Vein of Galen and internal cerebral vein. (e) Postoperative postcontrast T1 coronal, postsurgical changes demonstrating gross total resection of pineal region mass (white arrow). (f) Postoperative postcontrast T1 axial, postsurgical changes demonstrating residual tumor capsule (white arrow) attached to segments of the Vein of Galen and internal cerebral vein.
The lesion was resected through bilateral parietal-occipital stereotactic craniotomy (larger on the right side) utilizing a posterior right occipital interhemispheric and transtentorial approach as described in the previous case. Given the steep tentorial angle, prior surgical treatments this patient was subjected previously with subsequent morbidity, and goal to reach the most ventral aspect of the tumor without cerebellar retraction, the right occipital interhemispheric and transtentorial approach were selected over the supracerebellar-infratentorial option. The lesion was highly vascular, and we performed gradual central debulking with an ultrasonic aspirator with careful microdissection of the tumor capsule from vascular structures. A subtotal resection was performed, leaving the superior and left the aspect of the capsule, which was attached strongly to the Vein of Galen, bilateral veins of Rosenthal, and also toward the tectal plate of the midbrain. A right frontal ventriculostomy was placed preoperatively and removed 2 days after surgery.
On discharge, the patient’s baseline symptoms resolved and she was neurologically intact. Pathology confirmed a WHO Grade I fibrous meningioma. Follow-up magnetic resonance imaging (MRI) 8 months after surgery revealed the minimal residual presence and no signs of progression [
Patient 3
A 65-year-old male with a remote history of scalp melanoma had worsening headaches before admission. He was found to have a 3.2 cm × 2.9 cm × 3.2 cm posterior fossa lesion arising from the inferior surface of the left tentorial leaflet, associated with cerebellar edema, effacement of the fourth ventricle, and mild hydrocephalus [
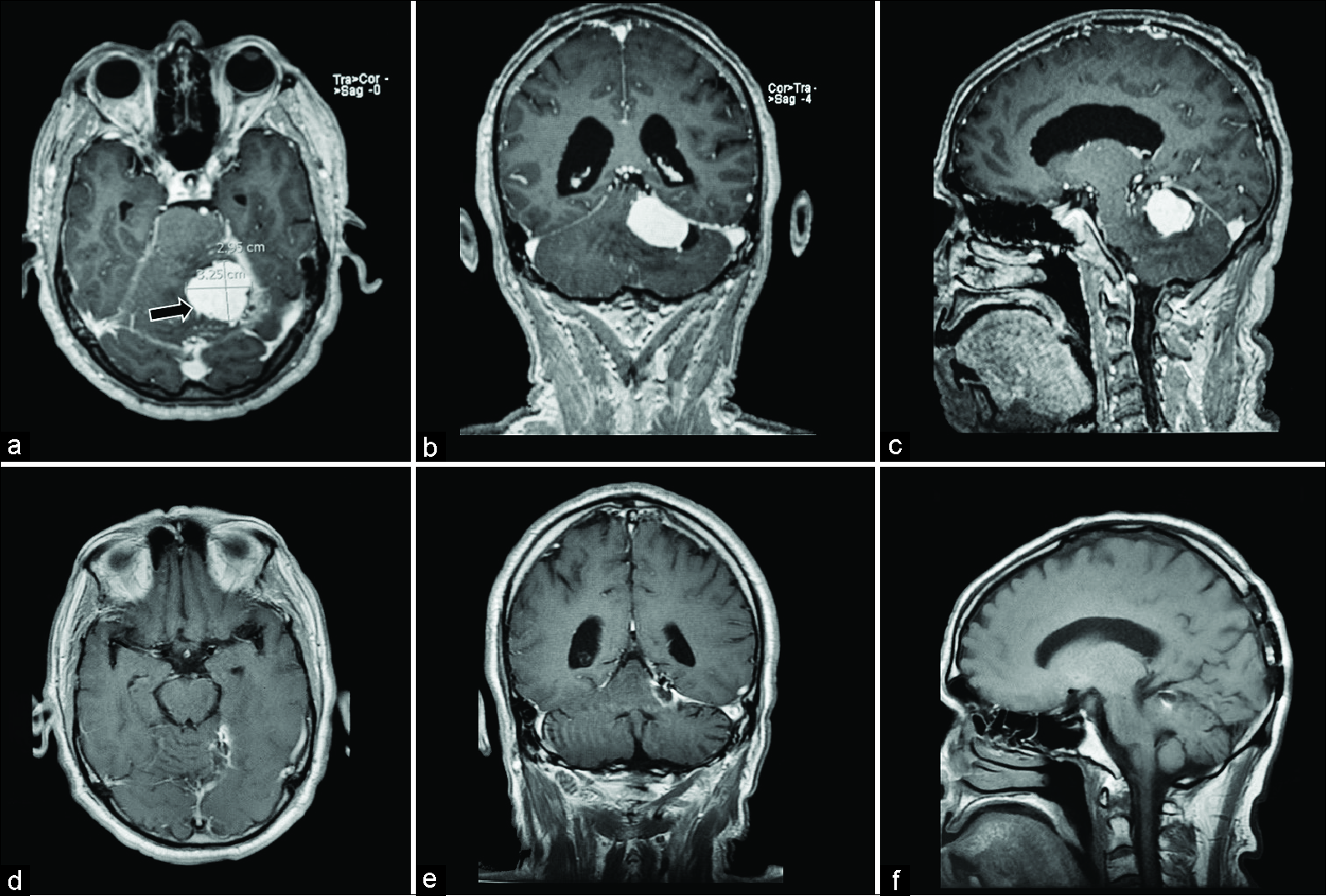
Figure 4:
Magnetic resonance images for patient 3. (a) Preoperative postcontrast T1 axial, homogeneously enhancing infratentorial paramedian lesion with high perilesional vascularity (black arrow). (b) Preoperative postcontrast T1 coronal, showing tentorial attachments. (c) Preoperative postcontrast T1 sagittal, showing a homogeneously enhancing mass with perilesional vascularity, and steep tentorial angle. (d) Postoperative (5 months) postcontrast T1 axial, demonstrating minimal tentorial enhancement and vascular clips artifact. (e) Postoperative postcontrast T1 coronal, demonstrating gross total resection and vascular clips artifact. (f) Postoperative noncontrast T1 sagittal, showing postsurgical changes.
The patient was kept in a lateral position using a beanbag, and the left side was kept down with cranial fixation, such that the sagittal plane was parallel to the floor. The lesion was resected through a bilateral parietal-occipital stereotactic craniotomy (larger on the left side), utilizing a posterior left occipital interhemispheric and transtentorial approach as described in the previous cases. A right frontal ventriculostomy was placed preoperatively, and we drained a total of 60 cc of CSF during the entire operation. After transtentorial exposure, we encountered a highly vascular and hemorrhagic tumor. We gradually debulked centrally with the ultrasonic aspirator and bipolar electrocoagulation. The largest arterial supply to the tumor, a branch of the superior cerebellar artery, was interrupted with a 7 mm titanium clip and the segment of the tentorium that was attached to the tumor was entirely resected, requiring hemoclips for hemostasis. The ventriculostomy was removed on postoperative day 2.
The patient was neurologically intact and discharged home on postoperative day 5. The pathology report confirmed a WHO Grade I hemangioblastoma, and an immediate postoperative MRI and another at 5 months postsurgery confirmed gross total resection [
Patient 4
A 47-year-old female presented with dysmetria and gait imbalance, with a history of metastatic breast cancer that was already managed at another institution with chemotherapy and whole brain radiation treatments for a superior vermian metastasis 3.1 cm × 3.0 cm × 3 cm [
Figure 5:
Magnetic resonance images for patient 4. (a) Preoperative postcontrast T1 axial, showing heterogeneously enhancing superior vermian lesion without hydrocephalus. (b) Preoperative postcontrast T1 coronal. (c) Preoperative postcontrast T1 sagittal, showing a heterogeneously enhancing mass with a displacement of tectal plate and steep tentorial angle. (d) Postoperative postcontrast T1 axial, demonstrating minimal surgical cavity and residual lesion. (e) Postoperative postcontrast T1 coronal, demonstrating partial resection. (f) Postoperative noncontrast T1 sagittal, demonstrating postsurgical changes.
A lumbar drain was placed, as no signs of obstructive hydrocephalus or increased pressure in the posterior fossa were encountered. The patient was kept in a lateral position using a beanbag and the right side was kept down with cranial fixation, with the sagittal plane parallel to the floor. The lesion was approached with a bilateral parietal-occipital stereotactic craniotomy (larger on the right side) utilizing a similar posterior right occipital interhemispheric and transtentorial approach. The lesion was centrally debulked using ultrasonic aspirator. The ventral part of the tumor was strongly attached to the superior cerebellar peduncles and midbrain and showed signs of calcification. We debulked as much as possible and eggshell the capsule of the tumor that was strongly attached to additional structures around the cerebellum, the vermis, and the brainstem, and proceeded to dissect centrally to the core. Overall, 25 cc of CSF was removed through the lumbar drain.
Postoperatively, the patient continued to experience dysmetria and gait imbalance. The lumbar drain was removed on postoperative day 2 and she was discharged to a rehabilitation center on postoperative day 13. Pathology reported metastatic adenocarcinoma with prominent necrosis and we are pending to start stereotactic radiation treatment for the residual lesion [
DISCUSSION
This report presents four cases of large posterior incisural space complex tumors that were successfully resected using a retractorless interhemispheric approach with good outcomes. Importantly, visual function was completely preserved in all patients. These findings indicate that in select patients, a retractorless interhemispheric approach may be advantageous in avoiding the risk of visual deficits, to overcome a considerable surgical challenge for the current standard techniques.
Falcotentorial meningioma resection approaches
Due to their complex nature and proximity to vital structures, several surgical approaches have been implemented in the treatment of falcotentorial meningiomas, including occipital interhemispheric transtentorial, infratentorial supracerebellar, biparietooccipital craniotomies, and supra/ infratentorial-trans-sinus approaches.[
For example, an occipital interhemispheric approach generally preserves the internal cerebral veins, Vein of Galen, and straight sinus.[
Visual deficits
Visual deficits are a frequent complication of the various approaches utilizing retractors to resect falcotentorial meningiomas, due to damage to the optic apparatus.[
Advantages of a retractorless posterior interhemispheric approach
The posterior interhemispheric approach has been used to access lesions in the pineal region, posterior incisural space, posterior region of third ventricle, and adjacent structures.[
CONCLUSION
The retractorless interhemispheric transtentorial approach presented here was successful in the resection of four large tumors in the posterior incisural space without damage to the optic apparatus or development of postoperative visual deficits. Together, this report suggests that a carefully executed retractorless approach should be considered for falcotentorial tumors, as well as large upper vermian lesions, as a means of preserving visual function, which currently poses a considerable surgical challenge.
Authors’ contributions
Conception and design: Lopez-Gonzalez, Patel. Acquisition of data: all authors analysis and interpretation of data: Lopez- Gonzalez, Patel. Drafting the manuscript: Lopez-Gonzalez, Patel. Critically revising the manuscript: all authors reviewed submitted version of the manuscript: all authors.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abecassis IJ, Hanak B, Barber J, Mortazavi M, Ellenbogen RG. A single-institution experience with pineal region tumors: 50 tumors over 1 decade. Oper Neurosurg (Hagerstown). 2017. 13: 566-75
2. Azab WA, Nasim K, Salaheddin W. An overview of the current surgical options for pineal region tumors. Surg Neurol Int. 2014. 5: 39-
3. Bassiouni H, Asgari S, König HJ, Stolke D. Meningiomas of the falcotentorial junction: Selection of the surgical approach according to the tumor type. Surg Neurol. 2008. 69: 339-49
4. Bassiouni H, Hunold A, Asgari S, Stolke D. Tentorial meningiomas: Clinical results in 81 patients treated microsurgically. Neurosurgery. 2004. 55: 108-16
5. Biroli A, Chiocchetta M, Gerosa M, Talacchi A. Surgical treatment of parasagittal and falcine meningiomas of the posterior third. Acta Neurochir (Wien). 2012. 154: 1987-95
6. Chi JH, Lawton MT. Posterior interhemispheric approach: Surgical technique, application to vascular lesions, and benefits of gravity retraction. Neurosurgery. 2006. 59: ONS41-9
7. Couldwell WT. Left occipital craniotomy for resection of falcotentorial meningioma. Neurosurg Focus. 2017. 43: V9-
8. Goto T, Ohata K, Morino M, Takami T, Tsuyuguchi N, Nishio A. Falcotentorial meningioma: Surgical outcome in 14 patients. J Neurosurg. 2006. 104: 47-53
9. Hernesniemi J, Romani R, Albayrak BS, Lehto H, Dashti R, Ramsey C. Microsurgical management of pineal region lesions: Personal experience with 119 patients. Surg Neurol. 2008. 70: 576-83
10. Hong CK, Hong JB, Park H, Moon JH, Chang JH, Lee KS. Surgical treatment for falcotentorial meningiomas. Yonsei Med J. 2016. 57: 1022-8
11. Ito Z. The microsurgical anterior interhemispheric approach suitably applied to ruptured aneurysms of the anterior communicating artery in the acute stage. Acta Neurochir (Wien). 1982. 63: 85-99
12. Kennedy BC, Bruce JN. Surgical approaches to the pineal region. Neurosurg Clin N Am. 2011. 22: 367-80
13. Liu JK. Combined bi-occipital suboccipital transsinus transtentorial approach for resection of a pineal region falcotentorial meningioma: Operative video and technical nuances. Neurosurg Focus. 2016. 40: V14-
14. Liu JK, Cohen MA. Endoscopic-assisted posterior interhemispheric retrocallosal transfalcine approach for microsurgical resection of a pineal region falcotentorial meningioma: Operative video and technical nuances. Neurosurg Focus. 2016. 40: V15-
15. Liyong S, Bao Y, Liang J, Li M, Ren J. Posterior interhemispheric transtentorial approach for resection of a meningioma at the posteromedial tentorial incisura. Neurosurg Focus. 2016. 40: V8-
16. Lopez-Gonzalez MA, Dolan E. Endodermal cyst in pineal region: Rare location. Surg Neurol Int. 2016. 7: S279-81
17. Lu ZF. Resection of suprasellar meningioma through interhemispheric approach. J Craniofac Surg. 2014. 25: 1302-4
18. Nanda A, Patra DP, Savardekar A, Maiti TK, Konar SK, Notarianni C. Tentorial meningiomas: Reappraisal of surgical approaches and their outcomes. World Neurosurg. 2018. 110: e177-e196
19. Nazzaro JM, Shults WT, Neuwelt EA. Neuro-ophthalmological function of patients with pineal region tumors approached transtentorially in the semisitting position. J Neurosurg. 1992. 76: 746-51
20. Nowak A, Dziedzic T, Czernicki T, Kunert P, Marchel A. Falcotentorial and velum interpositum meningiomas: Two distinct entities of the pineal region. Neurol Neurochir Pol. 2014. 48: 397-402
21. Oliveira J, Cerejo A, Silva PS, Polónia P, Pereira J, Vaz R. The infratentorial supracerebellar approach in surgery of lesions of the pineal region. Surg Neurol Int. 2013. 4: 154-
22. Ono M, Ono M, Rhoton AL, Barry M. Microsurgical anatomy of the region of the tentorial incisura. J Neurosurg. 1984. 60: 365-99
23. Quiñones-Hinojosa A, Chang EF, Chaichana KL, McDermott MW. Surgical considerations in the management of falcotentorial meningiomas: Advantages of the bilateral occipital transtentorial/transfalcine craniotomy for large tumors. Operative Neurosurgery. 2009. 64: 260-8
24. Quinones-Hinojosa A, Chang EF, McDermott MW. Falcotentorial meningiomas: Clinical, neuroimaging, and surgical features in six patients. Neurosurg Focus. 2003. 14: e11-
25. Raco A, Agrillo A, Ruggeri A, Gagliardi FM, Cantore G. Surgical options in the management of falcotentorial meningiomas: Report of 13 cases. Surg Neurol. 2004. 61: 157-64
26. Terasaka S, Asaoka K, Kobayashi H, Yamaguchi S. Anterior interhemispheric approach for tuberculum sellae meningioma. Neurosurgery. 2011. 68: 84-8