- Department of Neurosurgery, Philipps-University Marburg, Baldingerstraβe, Germany
- Department of Radiotherapy and Radiation Oncology, Philipps-University Marburg, Baldingerstraβe, Germany
Correspondence Address:
Mirza Pojskic
Department of Neurosurgery, Philipps-University Marburg, Baldingerstraβe, Germany
DOI:10.4103/sni.sni_228_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mirza Pojskic, Miriam H. A. Bopp, Markus Schymalla, Christopher Nimsky, Barbara Carl. Retrospective study of 229 surgically treated patients with brain metastases: Prognostic factors, outcome and comparison of recursive partitioning analysis and diagnosis-specific graded prognostic assessment. 24-Oct-2017;8:259
How to cite this URL: Mirza Pojskic, Miriam H. A. Bopp, Markus Schymalla, Christopher Nimsky, Barbara Carl. Retrospective study of 229 surgically treated patients with brain metastases: Prognostic factors, outcome and comparison of recursive partitioning analysis and diagnosis-specific graded prognostic assessment. 24-Oct-2017;8:259. Available from: http://surgicalneurologyint.com/surgicalint-articles/retrospective-study-of-229-surgically-treated-patients-with-brain-metastases-prognostic-factors-outcome-and-comparison-of-recursive-partitioning-analysis-and-diagnosis%e2%80%91specific-graded-progno/
Abstract
Background:Metastases are the most frequent tumors in the brain. Most often used scoring systems to predict the outcome are the RPA (Recursive Partitioning Analysis) classification and the DS-GPA (Diagnosis-Specific Graded Prognostic Assessment) score. The goal of our study was to determine prognostic factors which influence outcome in patients who undergo surgery for brain metastases and to compare different outcome scores.
Methods:Two hundred and twenty-nine patients who underwent surgery for brain metastases in our institution between January 2005 and December 2014 were included in the study. Patient data were evaluated retrospectively.
Results:The mean survival time was 19.2 months (median survival time, MST: 8 months), for patients with a single metastasis (n = 149) 17.6 months (MST: 8 months), and for patients with multiple metastases (n = 80) 17.9 months (MST: 6 months). Significant influence on MST had age P = 0.002), female sex (10 vs. 6 months, P P 70% (11 vs. 4 months, P P
Conclusion:Favorable factors for prolonged survival were KPS >70%, RPA Class I and II, age
Keywords: Brain metastasis, Diagnosis-Specific Graded Prognostic Assessment, Recursive Partitioning Analysis
INTRODUCTION
Almost 25% of all patients with oncological diseases present with cerebral metastases.[
Surgery and radiosurgery are established treatment strategies for patients with single brain metastasis, followed by radiation therapy.[
Median survival time (MST) in patients with brain metastases without any therapy is 1 month only and with steroids around 2 months. MST after WBRT is 3–6 months. MST after resection of brain metastasis is differently reported in literature in the range of between 6 and 17 months.[
Surgical resection plays an important role in relieving mass effects and decompressing eloquent areas of the brain causing improvement of the neurological status.[
The goal of our study was to determine prognostic factors which influence outcome in patients who undergo surgery for brain metastases and to compare different outcome scores.
MATERIALS AND METHODS
Two hundred and twenty-nine patients who underwent surgery for brain metastases in our institution between January 2005 and December 2014 were included in this study. The follow up period was extended to April 2017. Patient data were retrieved from charts and electronic databases. The patient data were evaluated retrospectively.
All patients were evaluated with respect to the following parameters: age, sex, primary tumor, presence of extracranial metastases assessed by contrast-enhanced computed tomography scan of thorax, abdomen, and pelvis, location of the metastasis (differentiating between supra- and infratentorial lesions as well as eloquent and non-eloquent region), number of metastases, number of resected lesions, MST, preoperative and postoperative Karnofsky Performance Score (KPS), RPA, DS-GPA, complications, postoperative radiotherapy, postoperative chemotherapy, and metastasis recurrence.
Two hundred and twenty-nine patients who underwent surgery for brain metastases in our institution between January 2005 and December 2014 were included in the study. Follow-up period ranged from 1 month to 126 months with a mean follow-up of 10.3 months. Until the end of the follow-up, 207 patients died and 22 patients (9.6%) were alive.
Surgery was performed for patients with symptomatic single or multiple brain metastases. The indications for surgery included large supra- and infratentorial space-occupying lesions and brain metastases of unknown primary at the time of the diagnosis (metastasis as the first symptom of the primary tumor).
Survival was calculated from the day of the resection of the brain metastasis until death or until the end of the follow-up period. Patients were followed by MRI at 3 months interval. Overall survival rates were calculated using the Kaplan–Meier method. Differences between the Kaplan–Meier curves were determined with the log-rank test (univariate analysis); only if Kaplan-Meier curves crossed the Tarone–Ware test was used. P values <0.05 were considered statistically significant. All statistical computations were performed using SPSS Statistics 23 (IBM, Germany).
To evaluate the diagnostic power of DS-GPA and RPA scoring systems with respect to survival, two Cox regressions were modeled. In the first Cox regression model, the predictor of survival is the DS-GPA score; in the second model, the predictor of survival is the RPA Score. The model accuracy is assessed by means of the fit statistic – 2 log likelihood and the overall score (Chi-square).
RESULTS
Patient characteristics
Two hundred and twenty-nine patients who underwent surgery for brain metastases in our institution between January 2005 and December 2014 were included in the study. There were 114 male (49.8%) and 115 female (50.1%) patients. Patient age ranged from 26 to 86 years, with the medium age being 59.7 years.
Follow-up period ranged from 1 month to 126 months with a mean follow-up of 10.3 months. Till the end of the follow-up 207 patients died and 22 patients (9.6%) were still alive.
One hundred and forty-nine patients (67 male and 82 female) or 65.1% underwent surgery for single metastasis, and 80 patients (47 male and 33 female) or 34.9% underwent surgery for multiple brain metastasis. Forty-one patients had two metastasis (12.0%), 10 had three metastases (4.4%), 7 had four (3.1%), and 22 more than four metastases (9.6%). Among 41 patients with two metastases, both lesions were resected in 9 patients and one lesion in 32 patients. From 10 patients with three metastases, in 1 patient all three metastases were resected, in 1 patient two out of three and in 8 patients only one metastasis. From 7 patients with four metastases, in 2 patients all four metastases were resected, in 1 patient two out of four, and in 4 patients one metastasis. From 22 patients with more than four metastases, all metastases were resected in 2 patients and in 20 patients only one metastasis was operated. The maximal number of metastases resected in 1 patient was five.
For 156 patients or 68.1%, surgery was performed due to a supratentorial lesion; in the remaining 73 cases or 31.9%, an infratentorial lesion was resected. In 91 cases or 39.7%, the lesions were located in an eloquent area, among them 35 operations or 15.3% were performed due to tumors located in the central region.
According to the primary tumor, we divided the patients into seven groups – lung cancer, breast cancer, melanoma, gastrointestinal tumors, renal carcinoma (including urothel carcinoma), carcinoma of unknown primary (CUP), and others. The most common primary tumors were lung cancer (SCLC and NSCLC, 86 or 37.5%), followed by breast cancer (50 or 21.9%). In 30 patients or 13.1%, tumors of the gastrointestinal tract were primary tumors (14 with rectal carcinoma, 8 with colon carcinoma, 2 each with hypopharynx carcinoma and esophagus carcinoma, and 1 each with hepatocellular, stomach, pancreas, and peritoneal carcinoma). Twenty-four patients or 10.5% had a melanoma as the primary tumor and 15 or 6.5% had renal carcinoma (renal cell carcinoma in 10 and urinary bladder urothel carcinoma in 5 patients). There were 6 patients with ovarian carcinoma (2.6%), four patients with carcinoma of unknown primary (1.74%), 3 patients with cervical cancer (1.31%), 2 patients each with testicular cancer and prostatic cancer (0.87% each), and 1 patient each with chloroma, endometrium carcinoma, vulvar cancer, follicular carcinoma of thyroid gland, malignant trophoblastosis, malignant peripheral nerve sheath tumor, and leiomyosarcoma (0.4% each). At the time of operation, 126 patients or 55% had extracranial metastases.
The most common symptoms at the time of presentation were signs of elevated intracranial pressure, including headache in 66 patients (28.8%), often combined with nausea and vomiting (51 cases or 22.3%). Sixty-five patients or 28.3% had a motor neurological deficit (monoparesis or hemiparesis). Speech disturbances (motoric, sensory, or global aphasia, usually in lesions near classical Broca or Wernicke area) occurred in 25 patients (10.9%) and visual problems (usually retro orbital lesions or lesions involving the primary visual cortex in the occipital lobe) in another 25 patients. Twenty-seven patients or 11.8% presented with seizures. In 27 cases, the patients were asymptomatic and the metastasis was discovered during staging for metastasis of the known primary tumor. In 9 patients or 3.93%, hydrocephalus occurred with the need of implantation of ventriculoperitoneal or ventriculoatrial shunt. In all of these cases, occlusive hydrocephalus occurred due to infratentorial metastasis and did not resolve after the resection of the lesion. In 13 patients or 5.67%, patients presented with intracerebral hemorrhage due to hemorrhage in the metastasis; in 5 cases the primary tumor was melanoma, in 4 cases renal carcinoma, in 2 cases breast cancer, and in 1 each prostate cancer and NSCLC. In 76 patients or 33.2%, the cerebral metastasis led to the first diagnosis of the primary tumor.
Mean survival time and median survival time
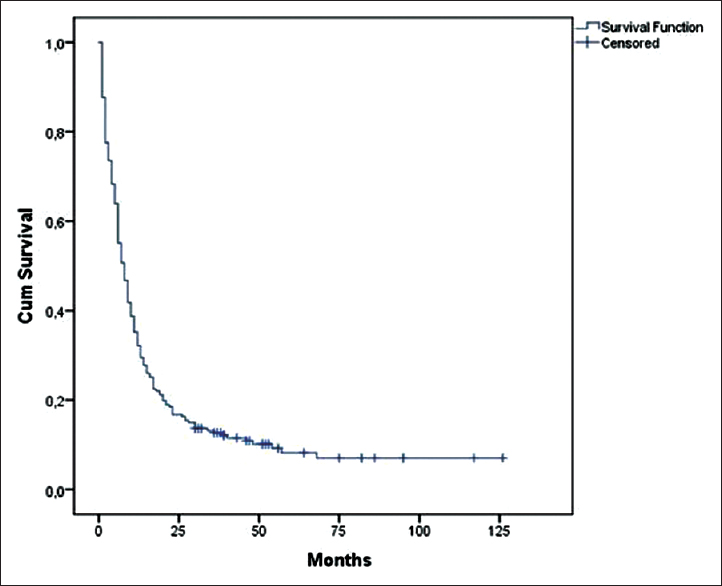
The mean survival time of the whole group was 19.2 months (SE 2.289 months, 95% CI lower bound 14.7, upper bound 23,7); MST was 8 months (SE 0.752 months, 95% CI lower bound 6.5, upper bound 9.4) [
Age
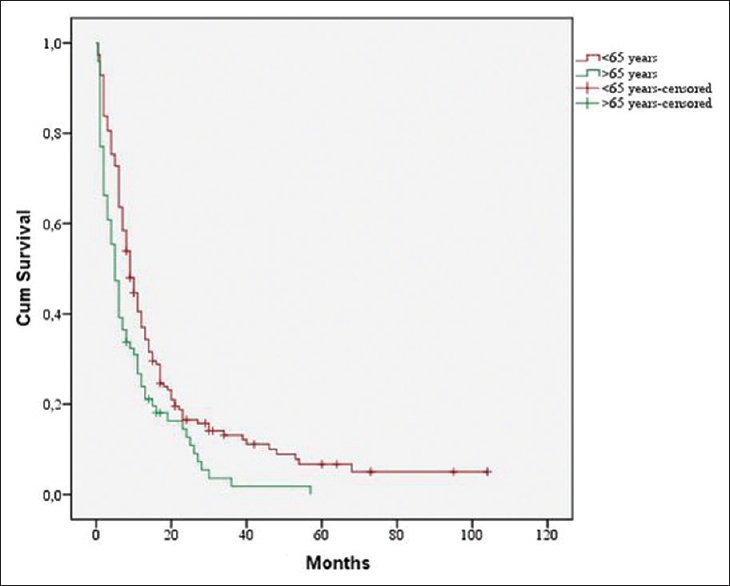
One hundred and thirty-seven patients or 59.8% were at the time of surgery younger than 65 years. Age <65 years had a significant influence on median survival time (9 months vs. 5 months, P = 0.002) [
Sex
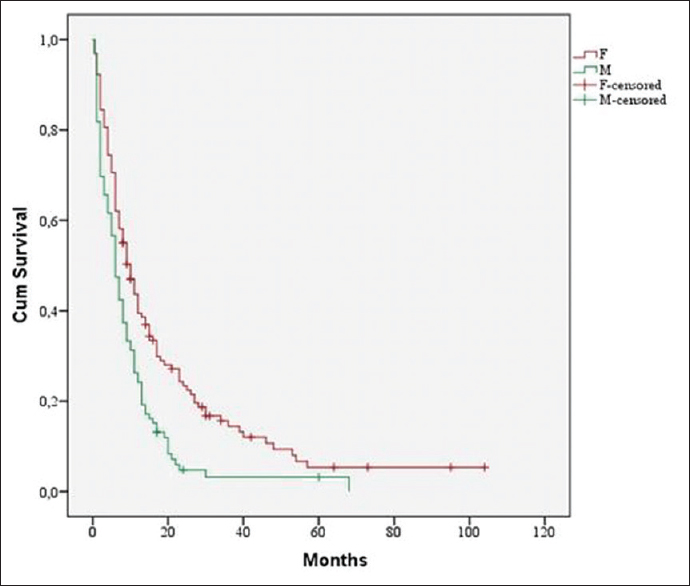
Female sex had a significant influence on median survival time (10 months vs. 6 months, P < 0.001) [
Karnofsky performance score
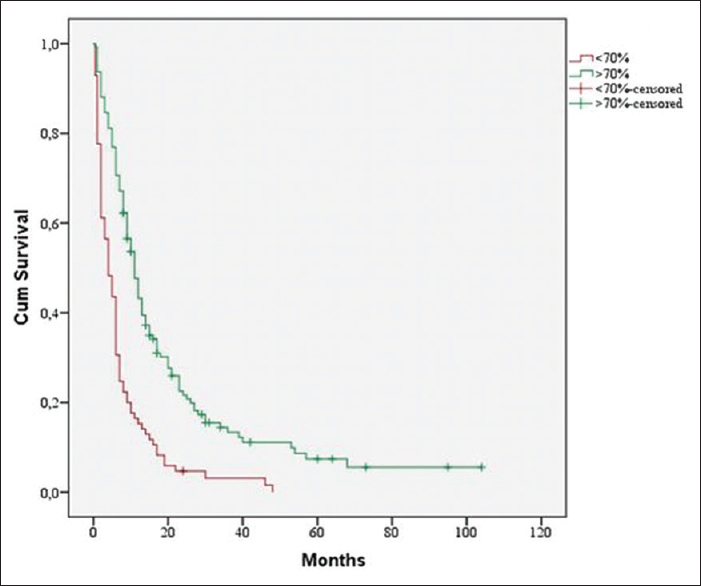
One hundred and forty-two patients or 62% had a preoperative KPS >70% and 87 or 38% had KPS <70%. Preoperative KPS >70% had a significant influence on median survival time. There is a strong correlation between preoperative and postoperative KPS. Thirty-eight percent of our patients had preoperatively a KPS <70%. Ninety-four percent of patients with a KPS <70% preoperatively had KPS <70% postoperatively. Among all the patients with a preoperative KPS >70%, 97% had a postoperative KPS >70% and only 3% deteriorated. A postoperative KPS >70% also showed significant correlation to prolonged MST (11 vs. 4 months, P < 0.001) [
Recursive partitioning analysis
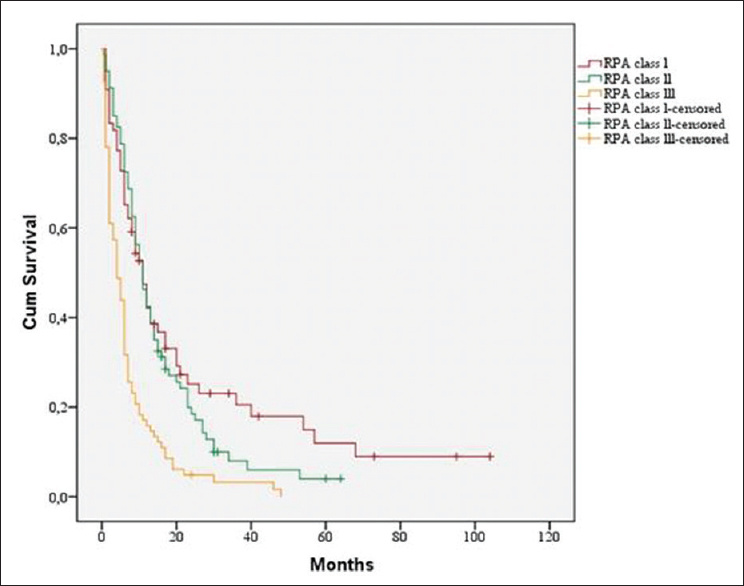
The patients with RPA Class I and II had the same median survival time (11 months). RPA Classes I and II showed significant correlation with a prolonged median survival time (11 months vs. 4 in RPA Class III, P < 0.001) [
Primary tumor and Diagnosis-Specific Graduated Prognostic Analysis
According to the primaries, the observed differences in the MST did not reach statistical significance. Patients with breast cancer metastases had the longest median survival time of 8 months. SCLC and NSCLC, melanoma, and renal cancer had a MST of 7 months each, gastrointestinal tumors, as well as group of other tumors 6 months and CUP 2 months (P = 0.030). The longest MST of 11 months had a subgroup of 32 patients with single metastasis of the breast cancer. All 32 patients underwent adjuvant radiotherapy.
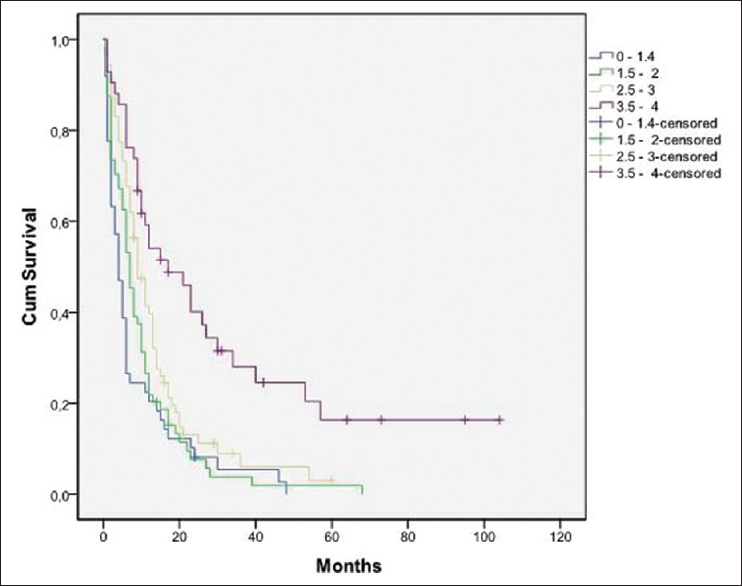
DS-GPA score showed a highly significant predictive power (P < 0.005). The patients with DS-GPA score of 0–1.4 had a median survival time of 4 months, score 1.5–2 MST of 7 months, the ones with a score 2.5–3 had MST of 9 and with DS-GPA score of 3.5–4 MST of 17 months. These differences were highly significant (P < 0.0001) [
Neurological outcome
One hundred and twenty-five patients (54.6%) had an acute neurological deficit before the surgery (motor deficit, speech deficit, visual disturbances). Among 125 patients which had a neurological deficit preoperatively, 98 patients or 78.2% improved after surgery, two worsened and 25 remained unchanged. Fifty-eight patients or 25.3% had a postoperative neurological deficit. Among these patients, in 33 patients, the neurological deficit improved after surgery and among 25 it remained unchanged.
Sixty-five patients had a preoperative motor neurological deficit (28.4%). In 19, the postoperative motor deficit didn’t resolve (29.2%). In 15 patients, the deficit was hemiparesis, and in 4 a monoparesis.
Aphasia occurred in 25 or 11% of patients. In 3 patients, the aphasia did not resolve after surgery.
Only 6 patients (2.9%) deteriorated neurologically after surgery. In 2 patients, hemiparesis and in 4 patients hemianopsia occurred after surgery as a new neurological deficit.
Radiotherapy
Postoperative radiotherapy was performed in 182 patients (79.4%). Postoperative whole brain radiotherapy (WBRT) with total dose of 30 Gray (Gy) was performed in 160 patients (69.9%). Twenty-two patients (9.6%) underwent fractionated stereotactic radiotherapy (FSRT) postoperatively (single dose 3 Gy, total dose 30–36 Gy). In the remaining 47 patients (20.5%) radiotherapy was not performed due to low KPS or due to patient decision against radiotherapy. In 8 patients, FSRT was performed due to recurrence after WBRT. Six patients who were treated with FSRT due to single metastasis experienced permanent growth after the treatment with neurological deficits due to edema and then underwent surgery.
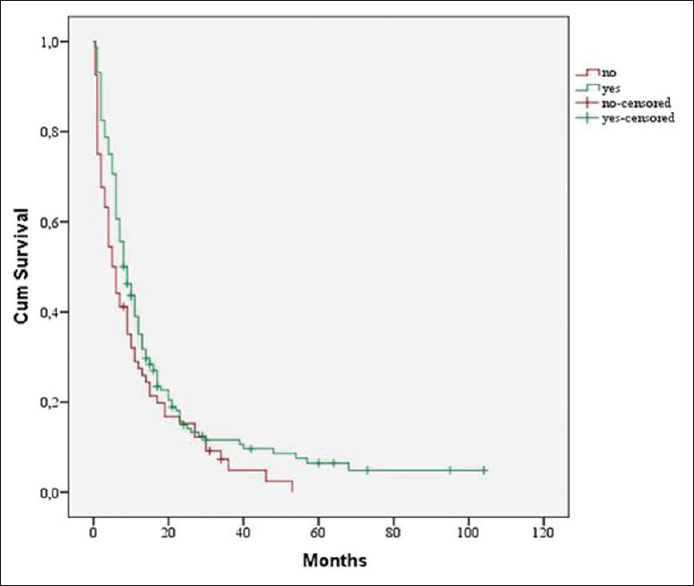
Postoperative radiotherapy had significant influence on MST compared to patients who did not receive any radiotherapy (8 months vs. 5 months, P < 0.02) [
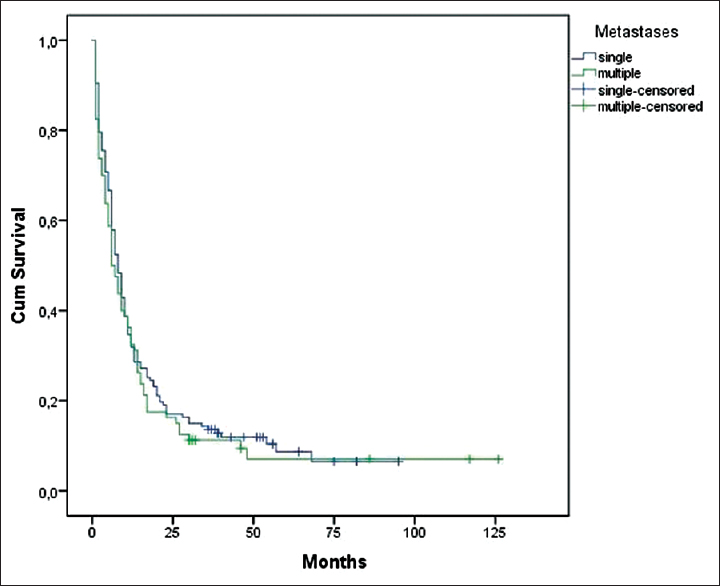
The number of metastases, postoperative chemotherapy, preoperative radiotherapy as well as presence of extracranial metastases were not significant in influencing median survival time (P > 0.05).
Complications and perioperative mortality
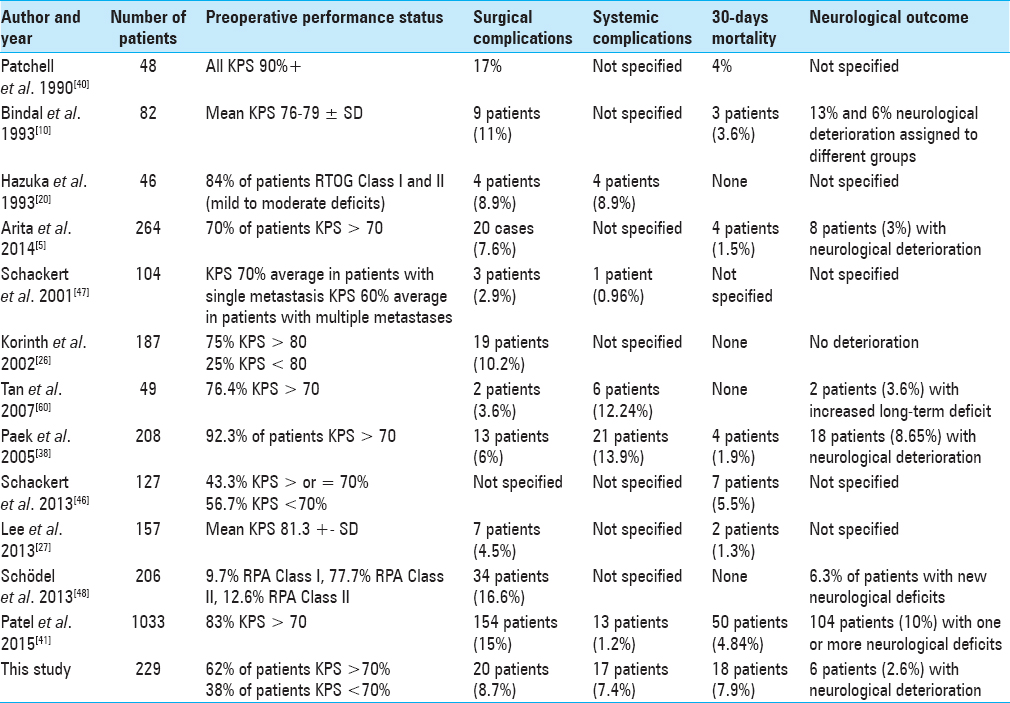
Eighteen patients (7.9%) died in the first 30 days after the surgery. Ten of these patients had a preoperative KPS <70% (RPA Class III). Causes of death were rapid progression of the primary disease (n = 8), sepsis due to pneumonia in mechanically ventilated patients (n = 6), and myocardial infarction with heart failure (n = 3). One patient died due to brainstem infarction as an operative complication after resection of an infratentorial metastasis.
We divided complications as surgical and nonsurgical. Surgical complications were divided into local and neurological. Nonsurgical complications were systemic. In 20 patients (8.7%), local complications leading to revision surgery occurred. In 16 patients, wound healing deficits occurred which needed to be reoperated (in 7 patients together with intracranial abscess or subdural empyema), in 3 patients cerebrospinal fluid (CSF) fistula, and postoperative hemorrhage in the resection cavity in 1 patient. Only six patients (2.6%) deteriorated neurologically after surgery. Systemic complications occurred in 17 patients (7.4%) and included pulmonary embolism (n = 5), pneumothorax (n = 2), sepsis due to pneumonia in mechanically ventilated patients (n = 6), myocardial infarction with heart failure (n = 3), and status epilepticus (n = 1).
Recurrence
Local recurrence occurred in 41 or 17.9% patients, and distant new metastases occurred in 39 or 17% of all patients. In 23 patients or 10%, both local and distant recurrence occurred. In case of recurrence, reoperation or FSRT were considered. Reoperation for recurrence was performed in five patients. These patients were not double counted in the study.
Significant factors for prolonged survival
Prognostic favorable factors for prolonged survival were KPS >70%, RPA Class I and II, age <65 years, female sex, DS-GPA Score of 2.5–3 and 3.5–4, and adjuvant WBRT. Patients with breast cancer metastases had the longest median survival time.
Comparison of RPA classification and DS-GPA classification
To evaluate the diagnostic power of DS-GPA and RPA class in respect of survival, two Cox regressions were modeled. RPA Classification was more accurate in predicting the outcome than the DS-GPA score. In both models the predictive power of two gradings is highly significant (P < 0.005), the RPA classification showed a better predictive power (-2 log likelihood = 1771. 235 and χ2 = 16.807).
DISCUSSION
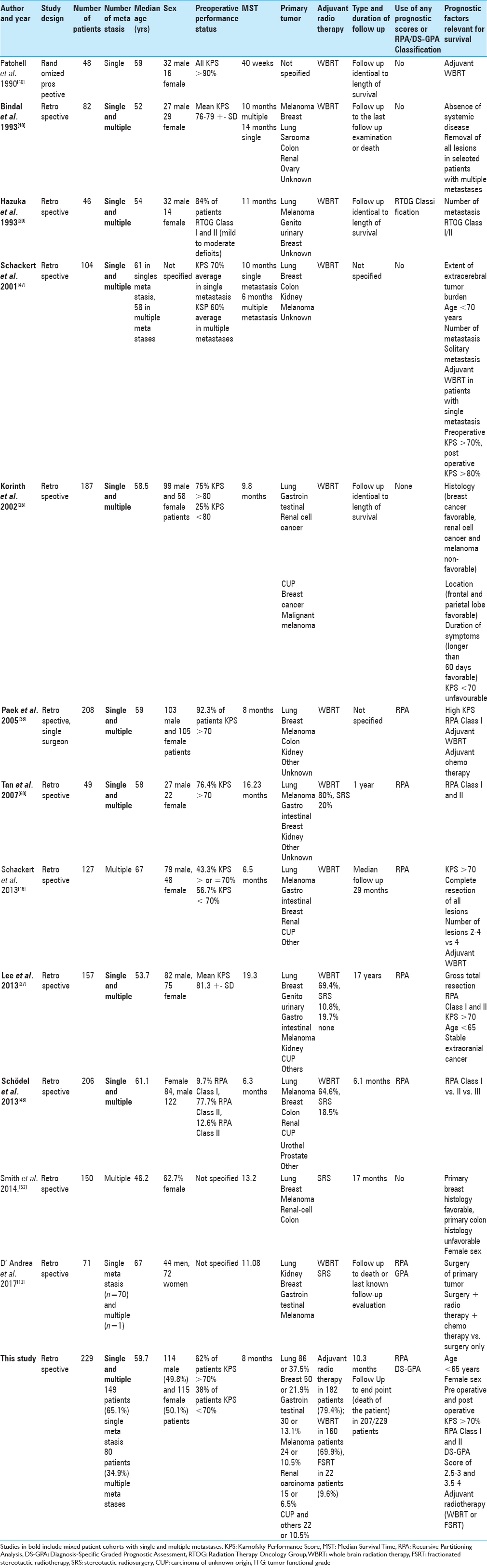
In the past 25 years, several studies have analyzed prognostic factors which influence survival in patients operated for brain metastasis.[
Table 2
Overview of studies which evaluated relevant prognostic factors in patients with surgically treated brain metastases with comparison of study design, number of patients, number of metastasis, median age, sex, preoperative performance status, MST, primary tumor, adjuvant radiotherapy, type and duration of follow up, use of any prognostic scores or RPA/DS-GPA Classification with overview of prognostic factors relevant for survival
Indications
Our patients consisted of a selected group judged as not being suitable for radiotherapy alone. We decided for the resection of the tumor when it offered a significant mass reduction to reduce intracranial pressure and gain time for adjuvant treatments. An important indication was also an unknown primary tumor. The indication for resection of infratentorial metastasis was given to avoid occlusion of the fourth ventricle and hydrocephalus. Supramarginal resection was performed, which in a recent study of Pessina et al. showed to be safe and effective for selected patients with large brain metastasis.[
Despite the advantages of SRS or radiotherapy as a local treatment, studies on surgical resection have demonstrated that surgery is even more beneficial for improving neurological status and survival.[
Median survival time
In our study, we dealt with two groups of patients, the group with single metastasis and the group with multiple metastases. So far in literature there are five major studies including patients both with single and multiple metastases evaluating outcome after surgery.[
Lee et al. reported a median survival in their surgical series of 19.3 months, 28.1 months in patients with no evidence of systemic disease, and 23.3 months in patients with stable disease.[
MST was 8 months for the entire group, and 8 months for the group of single metastasis and 6 months for the group of multiple metastases. MST in this study was larger than MST in the study of Schödel et al. (6.3 months)[
Age and gender
Age was very early recognized as an important factor in survival[
Recursive partitioning analysis
Comparing the MST according to RPA classification, our results were highly significant. An MST of 11 months for Class I and II and an MST of 4 months for Class III is longer than the MST predicted in the original paper of Gaspar et al. (7.1 vs. 4.2 vs. 2.3 for Class I, II, and III, respectively). This study has a larger percentage of patients who preoperatively belonged to RPA Class III than the studies of Schackert et al. and Tan et al.[
Primary tumor and Diagnosis-Specific Graduated Prognostic Analysis
Patients with breast cancer metastases had the longest median survival time of 8 months. This is significantly lower than the study of Smith et al. (22.9 months).[
According to the DS-GPA Classification, patients with a score of 0–1 had a longer MST than predicted by Sperduto et al. (4 months vs. 3–3.4 for different primary tumors), score 1.5–2 with MST of 7 months (compared to 7.7 in breast cancer, 7.3 in renal carcinoma, 5.5 in lung cancer, 4.7 melanoma, and 4.4 for GI cancers in the original paper of Sperduto et al.), score 2.5–3 had a MST of 9 (compared to 9.4 for lung cancer, 8.8 melanoma, 15.1 breast cancer, 11.3 renal cell carcinoma, 6.9 GI cancer) and with DS-GPA score of 3.5–4 MST of 10 months (less than predicted in all groups of primary tumors). As explained by Kondziolka et al.,[
When compared to DS-GPA Score, RPA Classification showed a better predictive power, although both scores had a highly significant predictive power. This is not to be misunderstood with importance of primary tumor diagnosis, which is not being taken into consideration in RPA classes. Prospective randomized trials are needed to be done to asses new prognostic scores which combine the parameters from RPA classification and DS-GPA score.
Prognostic indices have been utilized in different malignancies with the aim to improve the understanding of patients’ prognosis and aid the clinical and therapeutic decision making.[
In 2007, a new scoring system called the GPA was proposed. The GPA incorporated four factors: age, KPS, extra cranial metastases, and number of metastases[
A considerable variation in survival prediction was noted in the study by Kondziolka et al.,[
Single vs. multiple metastases
The number of metastases was not a significant factor which influenced prolonged median survival time. Smith et al. showed that the 1-year survival for patients with multiple intracranial metastases treated with resection followed with stereotactic radiosurgery is similar to established outcomes in patients with single brain metastasis.[
Hazuka et al.[
The importance of the actual number of metastases as a significant factor for prognosis was disputed in a recent review article.[
Neurological outcome
Surgical resection causes significant neurofunctional improvement in most patients with brain metastasis independent from RPA classification.[
Only 6 patients (2.6%) deteriorated neurologically after surgery. Korinth et al. and D’Andrea et al. report that there were no cases of neurological deterioration in their cohorts.[
13.1% of patients were asymptomatic. Although routine brain screening is not common, oncologists tend to obtain MRI on any sign of neurological symptoms. In the future, the inclusion of increasing numbers of asymptomatic brain metastases from screening may lead to a lead time bias for survival outcomes.[
Radiotherapy
Our study confirmed the importance of adjuvant radiotherapy after surgical resection of brain metastases. Postoperative radiotherapy was performed in 79.4% of patients and showed significant influence on MST compared to patients which did not receive radiotherapy (8 months vs. 5 months) [
Two randomized clinical trials have demonstrated that surgical resection is superior to WBRT only,[
When used as a primary treatment for solitary metastasis, radiosurgery has been associated with local tumor control rates of 73–94% and less morbidity than WBRT. Radiosurgery has also been shown to reduce local tumor recurrence following gross total resection of a single brain metastasis.[
In recent years, surgery and FSRT or radiosurgery alone largely replaced WBRT.[
In our study, only 15 patients or 7.28% underwent postoperative FSRT immediately following the operation. These patients had an MST of 15 months, although due to small sample and bias due to the fact that all these patients had a KPS 100% no comparison to the WBRT group is possible. SRS can lead to excellent tumor control and survival rates comparable to surgical evacuation,[
There are several questions on the issue that the patients who received postoperative radiotherapy had longer MST than the patients who did not receive the treatment, which due to the retrospective character of our study cannot be answered. Do these patients live longer because they develop less new metastases in the brain or because the operated and irradiated metastasis showed no recurrence or did the patients who received radiotherapy had controlled disease with less extra cranial metastases where the brain metastasis was not the immediate cause of death, remains to be evaluated in large prospective studies in the future.
Complications
Compared to other studies, we could show a low complication rate. Both neurological deterioration as well as local and systemic complications are lower than in the study of Paek et al., Schackert et al., Patel et al., and Bindal et al.[
Eighteen patients or 7.9% died in the period of 30 days after the surgery, which is higher than in the series of Bindal et al., Hazuka et al., Arita et al., Schackert et al., Paek et al., and Patel et al.[
The shortcoming of the study is its retrospective design. Because of our limited sample size, our study may be too underpowered to detect differences between subgroups. Despite these limitations, this study is, to our knowledge, the first single-institution analysis of survival following resection of brain metastasis which is reevaluating and comparing RPA Classes and DS-GPA Score. Our findings show the importance of surgery, as well as the importance of adjuvant radiotherapy in assessing the prognosis after surgery, but also indicate the shortcomings of the DS-GPA Score, as the RPA Classification showed a better predictive power. Future prospective randomized studies are needed to establish the efficacy of the existing treatments and to lead to improvement of estimation of survival of each patient and in addition to it to optimize individual therapy and increase survival.
CONCLUSION
Prognostic favorable factors for prolonged survival were KPS >70%, RPA Class I and II, age <65 years, female sex, DS-GPA Score of 2.5–3 and 3.5–4 and adjuvant radiotherapy (WBRT or FSRT). Patients with breast cancer metastases had a longer MST compared to other primary tumors, although these differences were not statistically significant. Prospective randomized trials are needed to be done to asses new prognostic scores which combine the parameters from the RPA Classification and the DS-GPA Score.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agboola O, Benoit B, Cross P, Da Silva V, Esche B, Lesiuk H. Prognostic factors derived from recursive partition analysis (RPA) of Radiation Therapy Oncology Group (RTOG) brain metastases trials applied to surgically resected and irradiated brain metastatic cases. Int J Radiat Oncol Biol Phys. 1998. 42: 155-9
2. Al-Shamy G, Sawaya R. Management of brain metastases: The indispensable role of surgery. J Neurooncol. 2009. 92: 275-82
3. Antoni D, Clavier JB, Pop M, Benoît C, Lefebvre F, Noël G. An institutional retrospective analysis of 93 patients with brain metastases from breast cancer: Treatment outcomes, diagnosis-specific prognostic factors. Int J Mol Sci. 2012. 13: 16489-99
4. Aoyama H, Tago M, Kato N, Toyoda T, Kenjyo M, Hirota S. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys. 2007. 68: 1388-95
5. Arita H, Narita Y, Miyakita Y, Ohno M, Sumi M, Shibui S. Risk factors for early death after surgery in patients with brain metastases: Reevaluation of the indications for and role of surgery. J Neurooncol. 2014. 116: 145-52
6. Aupérin A, Arriagada R, Pignon JP, Le Péchoux C, Gregor A, Stephens RJ. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999. 341: 476-84
7. Barnholtz-Sloan JS, Yu C, Sloan AE, Vengoechea J, Wang M, Dignam JJ. A nomogram for individualized estimation of survival among patients with brain metastasis. Neuro Oncol. 2012. 14: 910-8
8. Bhatnagar AK, Flickinger JC, Kondziolka D, Lunsford LD. Stereotactic radiosurgery for four or more intracranial metastases. Int J Radiat Oncol Biol Phys. 2006. 64: 898-903
9. Bhatnagar AK, Kondziolka D, Lunsford LD, Flickinger JC. Recursive partitioning analysis of prognostic factors for patients with four or more intracranial metastases treated with radiosurgery. Technol Cancer Res Treat. 2007. 6: 153-60
10. Bindal RK, Sawaya R, Leavens ME, Lee JJ. Surgical treatment of multiple brain metastases. J Neurosurg. 1993. 79: 210-6
11. Borgelt B, Gelber R, Kramer S, Brady LW, Chang CH, Davis LW. The palliation of brain metastases: Final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1980. 6: 1-9
12. Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer. 1954. 7: 682-9
13. D’Andrea G, Palombi L, Minniti G, Pesce A, Marchetti P. Brain Metastases: Surgical Treatment and Overall Survival. World Neurosurg. 2017. 97: 169-77
14. Elliott RE, Rush S, Morsi A, Mehta N, Spriet J, Narayana A. Neurological complications and symptom resolution following Gamma Knife surgery for brain metastases 2 cm or smaller in relation to eloquent cortices. J Neurosurg. 2010. 113: 53-64
15. Esmaeilzadeh M, Majlesara A, Faridar A, Hafezi M, Hong B, Esmaeilnia-Shirvani H. Brain metastasis from gastrointestinal cancers: A systematic review. Int J Clin Pract. 2014. 68: 890-9
16. Gaspar LE, Scott C, Murray K, Curran W. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000. 47: 1001-6
17. Gavrilovic IT, Posner JB. Brain metastases: Epidemiology and pathophysiology. J Neurooncol. 2005. 75: 5-14
18. Han MS, Moon KS, Lee KH, Cho SB, Lim SH, Jang WY. Brain metastasis from hepatocellular carcinoma: The role of surgery as a prognostic factor. BMC Cancer. 2013. 13: 567-
19. Hara Y, Sakurai K, Adachi K, Fujiwara A, Ono Y, Nagashima S. [Examination of the Cases Given Primary Tumor Resection after Systemic Therapy for Metastatic Breast Cancer]. Gan To Kagaku Ryoho. 2015. 42: 1506-8
20. Hazuka MB, Burleson WD, Stroud DN, Leonard CE, Lillehei KO, Kinzie JJ. Multiple brain metastases are associated with poor survival in patients treated with surgery and radiotherapy. J Clin Oncol. 1993. 11: 369-73
21. Jagannathan J, Yen CP, Ray DK, Schlesinger D, Oskouian RJ, Pouratian N. Gamma Knife radiosurgery to the surgical cavity following resection of brain metastases. J Neurosurg. 2009. 111: 431-8
22. Kocher M, Soffietti R, Abacioglu U, Villà S, Fauchon F, Baumert BG. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J Clin Oncol. 2011. 29: 134-41
23. Kocher M, Wittig A, Piroth MD, Treuer H, Seegenschmiedt H, Ruge M. Stereotactic radiosurgery for treatment of brain metastases. A report of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlenther Onkol. 2014. 190: 521-32
24. Kondziolka D, Kalkanis SN, Mehta MP, Ahluwalia M, Loeffler JS. It is time to reevaluate the management of patients with brain metastases. Neurosurgery. 2014. 75: 1-9
25. Kondziolka D, Parry PV, Lunsford LD, Kano H, Flickinger JC, Rakfal S. The accuracy of predicting survival in individual patients with cancer. J Neurosurg. 2014. 120: 24-30
26. Korinth MC, Delonge C, Hütter BO, Gilsbach JM. Prognostic factors for patients with microsurgically resected brain metastases. Onkologie. 2002. 25: 420-5
27. Lee CH, Kim DG, Kim JW, Han JH, Kim YH, Park CK. The role of surgical resection in the management of brain metastasis: A 17-year longitudinal study. Acta Neurochir (Wien). 2013. 155: 389-97
28. Linskey ME, Andrews DW, Asher AL, Burri SH, Kondziolka D, Robinson PD. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010. 96: 45-68
29. Mathieu D, Kondziolka D, Flickinger JC, Fortin D, Kenny B, Michaud K. Tumor bed radiosurgery after resection of cerebral metastases. Neurosurgery. 2008. 62: 817-
30. Matsunaga S, Shuto T, Kawahara N, Suenaga J, Inomori S, Fujino H. Gamma Knife surgery for metastatic brain tumors from primary breast cancer: Treatment indication based on number of tumors and breast cancer phenotype. J Neurosurg. 2010. 113: 65-72
31. Matsuo S, Watanabe J, Mitsuya K, Hayashi N, Nakasu Y, Hayashi M. Brain metastasis in patients with metastatic breast cancer in the real world: A single-institution, retrospective review of 12-year follow-up. Breast Cancer Res Treat. 2017. p.
32. Mintz AH, Kestle J, Rathbone MP, Gaspar L, Hugenholtz H, Fisher B. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer. 1996. 78: 1470-6
33. Muacevic A, Wowra B, Siefert A, Tonn JC, Steiger HJ, Kreth FW. Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: A randomized controlled multicentre phase III trial. J Neurooncol. 2008. 87: 299-307
34. Mut M. Surgical treatment of brain metastasis: A review. Clin Neurol Neurosurg. 2012. 114: 1-8
35. Narita Y, Shibui S. Strategy of surgery and radiation therapy for brain metastases. Int J Clin Oncol. 2009. 14: 275-80
36. Nieder C, Mehta MP. Prognostic indices for brain metastases--usefulness and challenges. Radiat Oncol. 2009. 4: 10-
37. Obermueller T, Schaeffner M, Gerhardt J, Meyer B, Ringel F, Krieg SM. Risks of postoperative paresis in motor eloquently and non-eloquently located brain metastases. BMC Cancer. 2014. 14: 21-
38. Paek SH, Audu PB, Sperling MR, Cho J, Andrews DW. Reevaluation of surgery for the treatment of brain metastases: Review of 208 patients with single or multiple brain metastases treated at one institution with modern neurosurgical techniques. Neurosurgery. 2005. 56: 1021-
39. Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA. 1998. 280: 1485-9
40. Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990. 322: 494-500
41. Patel AJ, Suki D, Hatiboglu MA, Rao VY, Fox BD, Sawaya R. Impact of surgical methodology on the complication rate and functional outcome of patients with a single brain metastasis. J Neurosurg. 2015. 122: 1132-43
42. Patil CG, Pricola K, Sarmiento JM, Garg SK, Bryant A, Black KL. Whole brain radiation therapy (WBRT) alone versus WBRT and radiosurgery for the treatment of brain metastases. Cochrane Database Syst Rev. 2012. p. CD006121-
43. Pessina F, Navarria P, Cozzi L, Ascolese AM, Maggi G, Rossi M. Role of Surgical Resection in Patients with Single Large Brain Metastases: Feasibility, Morbidity, and Local Control Evaluation. World Neurosurg. 2016. 94: 6-12
44. Redmond AJ, Diluna ML, Hebert R, Moliterno JA, Desai R, Knisely JP. Gamma Knife surgery for the treatment of melanoma metastases: The effect of intratumoral hemorrhage on survival. J Neurosurg. 2008. 109: 99-105
45. Sahgal A, Aoyama H, Kocher M, Neupane B, Collette S, Tago M. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: Individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2015. 91: 710-7
46. Schackert G, Lindner C, Petschke S, Leimert M, Kirsch M. Retrospective study of 127 surgically treated patients with multiple brain metastases: Indication, prognostic factors, and outcome. Acta Neurochir (Wien). 2013. 155: 379-87
47. Schackert G, Steinmetz A, Meier U, Sobottka SB. Surgical management of single and multiple brain metastases: Results of a retrospective study. Onkologie. 2001. 24: 246-55
48. Schödel P, Schebesch KM, Brawanski A, Proescholdt MA. Surgical resection of brain metastases-impact on neurological outcome. Int J Mol Sci. 2013. 14: 8708-18
49. Schöggl A, Kitz K, Reddy M, Wolfsberger S, Schneider B, Dieckmann K. Defining the role of stereotactic radiosurgery versus microsurgery in the treatment of single brain metastases. Acta Neurochir (Wien). 2000. 142: 621-6
50. Shimony N, Shofty B, Harosh CB, Sitt R, Ram Z, Grossman R. Surgical Resection of Cerebral Metastases Leads to Faster Resolution of Peritumoral Edema than Stereotactic Radiosurgery: A Volumetric Analysis. Ann Surg Oncol. 2016. p.
51. Shu HK, Sneed PK, Shiau CY, McDermott MW, Lamborn KR, Park E. Factors influencing survival after gamma knife radiosurgery for patients with single and multiple brain metastases. Cancer J Sci Am. 1996. 2: 335-42
52. Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007. 357: 664-72
53. Smith TR, Lall RR, Abecassis IJ, Arnaout OM, Marymont MH, Swanson KR. Survival after surgery and stereotactic radiosurgery for patients with multiple intracranial metastases: Results of a single-center retrospective study. J Neurosurg. 2014. 121: 839-45
54. Sperduto PW. What is your patient's GPA and why does it matter? Managing brain metastases and the cost of hope. Int J Radiat Oncol Biol Phys. 2010. 77: 643-4
55. Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: An analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008. 70: 510-4
56. Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: A multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010. 77: 655-61
57. Sperduto PW, Kased N, Roberge D, Chao ST, Shanley R, Luo X. The effect of tumor subtype on the time from primary diagnosis to development of brain metastases and survival in patients with breast cancer. J Neurooncol. 2013. 112: 467-72
58. Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X. Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012. 30: 419-25
59. Sperduto PW, Shanley R, Luo X, Andrews D, Werner-Wasik M, Valicenti R. Secondary analysis of RTOG 9508, a phase 3 randomized trial of whole-brain radiation therapy versus WBRT plus stereotactic radiosurgery in patients with 1-3 brain metastases; poststratified by the graded prognostic assessment (GPA). Int J Radiat Oncol Biol Phys. 2014. 90: 526-31
60. Tan TC, Black PM. Image-guided craniotomy for cerebral metastases: Techniques and outcomes. Neurosurgery. 2007. 61: 349-
61. Tsao M, Xu W, Sahgal A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer. 2012. 118: 2486-93
62. Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH. Treatment of single brain metastasis: Radiotherapy alone or combined with neurosurgery?. Ann Neurol. 1993. 33: 583-90
63. Venur VA, Ahluwalia MS. Prognostic scores for brain metastasis patients: Use in clinical practice and trial design. Chin Clin Oncol. 2015. 4: 18-
64. Walker AE, Robins M, Weinfeld FD. Epidemiology of brain tumors: The national survey of intracranial neoplasms. Neurology. 1985. 35: 219-26
65. Weidle UH, Niewöhner J, Tiefenthaler G. The Blood-Brain Barrier Challenge for the Treatment of Brain Cancer, Secondary Brain Metastases, and Neurological Diseases. Cancer Genomics Proteomics. 2015. 12: 167-77
66. Wroński M. [Surgical treatment of brain metastases from non-microcellular lung cancer]. Neurol Neurochir Pol. 1992. 26: 837-44
67. Wroński M, Arbit E, Burt M, Galicich JH. Survival after surgical treatment of brain metastases from lung cancer: A follow-up study of 231 patients treated between 1976 and 1991. J Neurosurg. 1995. 83: 605-16
68. Wroński M, Arbit E, Burt M, Perino G, Galicich JH, Brennan MF. Resection of brain metastases from sarcoma. Ann Surg Oncol. 1995. 2: 392-9
69. Wroński M, Arbit E, Russo P, Galicich JH. Surgical resection of brain metastases from renal cell carcinoma in 50 patients. Urology. 1996. 47: 187-93
70. Wroński M, Lederman G. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer. 1997. 80: 1002-4