- Department of Neurosurgery, Sher-I-Kashmir Institute of Medical Sciences, Srinagar, Jammu and Kashmir, India
Correspondence Address:
Arif Hussain Sarmast
Department of Neurosurgery, Sher-I-Kashmir Institute of Medical Sciences, Srinagar, Jammu and Kashmir, India
DOI:10.4103/2152-7806.172533
Copyright: © 2015 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kirmani AR, Sarmast AH, Bhat AR. Role of external ventricular drainage in the management of intraventricular hemorrhage; its complications and management. Surg Neurol Int 23-Dec-2015;6:188
How to cite this URL: Kirmani AR, Sarmast AH, Bhat AR. Role of external ventricular drainage in the management of intraventricular hemorrhage; its complications and management. Surg Neurol Int 23-Dec-2015;6:188. Available from: http://surgicalneurologyint.com/surgicalint_articles/role-of-external-ventricular-drainage-in-the-management-of-intraventricular-hemorrhage-its-complications-and-management/
Abstract
Background:External ventricular drainage (EVD) is the procedure of choice for the treatment of acute hydrocephalus and increased intracranial pressure in patients of subarachnoid hemorrhage (SAH) and intracerebral hemorrhage with hydrocephalus and its sequelae. We evaluated the use of EVD in patients of SAHs (spontaneous/posttraumatic with/without hydrocephalus), hypertensive intracerebral bleeds with interventricular extensions, along with evaluation of the frequency of occurrence of complications of the procedure, infectious and noninfectious, and their management.
Methods:During the period of 2½ years, between September 2012 and February 2015, 130 patients were subjected to external drainage procedure and were prospectively enrolled in this study. Information was collected on each patient regarding age, sex, diagnosis, underlying illness, secondary complications, other coexisting infections, use of systemic steroids, antibiotic treatment (systemic and intraventricular), and whether any other neurosurgical procedures were performed within 2 weeks of EVD insertion or any time the duration of ventriculostomy.
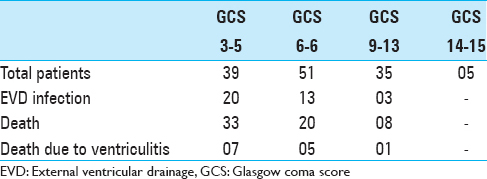
Results:The study population of 130 patients underwent a total of 193 ventriculostomies. Thirty-six patients had ventriculostomy infection (27.6%). Evaluation of the use of EVD was done by comparing preoperative and postoperative grading scores. Forty-nine patients survived and improved their score from Grade 3–5 to Grade 2–4. Twenty-nine patients were moderately disable, 16 were severely disable, and 5 were left in the vegetative state. Evaluation of outcome of patients revealed that there was an overall mortality of 61 (46.9%) patients both in the acute phase and later. 33 of the 39 patients having Glasgow Coma Score (GCS) 3–5 at the time of EVD insertion expired, as against 20 of the 51 patients in GCS 6–8. Patients in GCS 9–12 had an even better outcome, with 8 of the 35 patients in this group expiring.
Conclusions:The use of EVD should be undertaken only in situation where it is absolutely necessary and ventriculostomy should be kept only for the duration required, and this should be monitored on a daily basis, given the exponential increase in infection after 5 days.
Keywords: External ventricular drainage, Glasgow Coma Score, ventriculostomy
INTRODUCTION
Ventricular catheter placement is being increasing used in neurosurgery both as tool for monitoring of intracranial pressure (ICP)[
Despite the availability of high-potency antibiotics and closed drainage systems, the infection rate of ventricular catheters remains high; infection rates of 0–40% have been reported.[
MATERIALS AND METHODS
All patients admitted to the neurosurgical service at the Sher-I-Kashmir Institute of Medical Sciences, Soura, Srinagar, Jammu and Kashmir who underwent a percutaneous ventriculostomy between September 2012 and February 2015 were prospectively enrolled in this study as per our selection criteria given later. During this period of 2½ years, 130 patients were subjected to external drainage procedure.
The insertion of the ventricular catheter was done generally in the operation room under strict asepsis. While as only few cases were done as bedside procedures, in extremely sick patients, on a ventilator, in Neurosurgical Intensive Care Unit. Systemic antibiotics were given in all cases after the insertion of an EVD prophylactically. Intraventricular antibiotics were given only when clinically indicated (documented ventricular infection). The treatment of postoperative ventriculitis would be removal and/or change of catheter, systemic and intraventricular antibiotic therapy. Catheter change was undertaken in the event of catheter blockage any time and even for otherwise functioning noninfected catheter at varying intervals, most commonly between 5th and 8th days of insertion.
Information was collected on each patient regarding age, sex, diagnosis, underlying illness, secondary complications, other coexisting infections, use of systemic steroids, antibiotic treatment (systemic and intraventricular), and whether any other neurosurgical procedures were performed within 2 weeks of EVD insertion or any time the duration of ventriculostomy. CSF studies were done at least once in every 48 h and included cytology, culture, and biochemical analysis. Data regarding above CSF parameters were collected for each patient, besides maintaining a record of number of catheter changes, total duration, duration of each catheter, and time of onset of infection, as well as resolution of any EVD associated infections.
The infection was defined prior to undertaking this study as: A positive CSF culture or a combination of CSF sugar <15 mg/dl and pleocytosis at least 50 leukocytes with polymorphs more than 25. Pleocytosis was also taken to be significant in the presence of large numbers of red blood cells (RBCs) if RBC/white blood cell ratio was <500;1.
The presence of intraventricular hemorrhage (IVH) was noted radiologically, as well as clinically bedside by the appearance of blood in the external tubing. Computed tomography scan was done, both preoperatively, as well as postoperatively, for detection of any intraprocedural hemorrhagic complications and comparison of the size of ventricles, after drainage.
The patient with following criteria were included in the study:
Patients with IVH and ventriculomegaly having a deteriorating level of consciousness Patient with low Glasgow Coma Score (GCS), unstable vital parameters (hemodynamic or respiratory instability), and ventriculomegaly required bedside EVD placement Patient undergoing surgery for intraventricular and paraventricular tumors, as a prophylactic measure to avoid postoperative hydrocephalus and simultaneously allow ICP monitoring. The procedures were essential especially if the hemostasis at the time of definitive surgery was less than satisfactory.
Patient not included in the study were:
Those undergoing exteriorization of a previously performed shunt because most of them would have shunt infection although they may have undergone insertion of an EVD later Those patients having evidence of infection in the first stem cell factor sample obtained on EVD insertion Those having evidence of meningitis more than 7 days after removal of EVD.
The analysis of data was done using standard database techniques from which data were later converted to purely numeric format, for analysis with Statistix, version. 4.0, (Analytical Software, Tallahassee, Florida, USA) (variances, frequency distribution, and test of significances and BDMP survival analysis, univariate, and multivariate analysis).
RESULTS
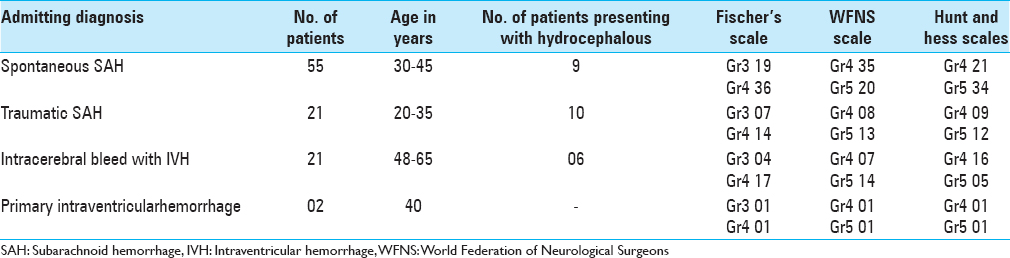
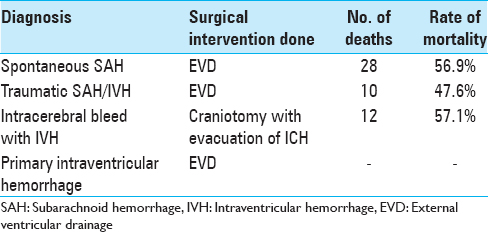
The study population consisted of 130 patients, who underwent a total of 193 ventriculostomies admitted over a period of 2½ years. The age distribution of patients was varied spread over all age groups, 0–10 years (14), 11–20 years (22), 21–30 years (41), 31–40 years (25), 41–50 (12), 51–60 (10), and above 60 years (06). Majority of patients who were subjected to EVD had spontaneous subarachnoid hemorrhage (SAH) with IVH (55), followed by posttraumatic IVH (23), hypertensive bleed with IVH (21), hydrocephalus (16), and hydrocephalus associated with deep-seated paraventricular tumors (15). All the patients were graded using grading scale devised by World Federation of Neurological Surgeons (WFNS), Hunt and Hess grading system, and Fischer's grading scale and the patients were categorized. The importance of assessing the neurological condition of patients after SAH lies in the prediction of outcome. Most of our patients were sick and were categorized under Grades 3, 4, and 5 according to WFNS and Hunt and Hess grading system. According to Fischer's scale, most of the patients fell in Group 3 and 4. All patients (25) with acute ventricular dilatation with increased ICP were subjected to EVD. Patients with associated intracerebral hematomas were subjected to craniotomy with the evacuation of hematoma, followed by an EVD to drain the intraventricular component of SAH. EVD was kept in place, until the time draining CSF was clear and the neurological condition would improve. The recommendation of the short-term use of EVD was followed (4–7 days) in this study. Prolonged use is associated with the risk of rebleeding, infection, increased morbidity and mortality. Evaluation of the use of EVD was done by comparing preoperative and postoperative grading scores. Forty-nine patients survived and improved their score from Grade 3–5 to Grade 2–4. Morbidity of the patients was assessed, and they were grouped as moderate disability, severe disability, and vegetative state on the criteria laid down by Glasgow Outcome Scale. Twenty-nine patients were moderately disable, 16 patients were severely disable, and 5 patients were left in a vegetative state as depicted in Tables
A total of 36 patients had ventriculostomy infection, implying an overall infection rate of 27.6%. Among the infected 36 patients, cultures were positive in 29 patients (80.5%). The infections were polymicrobial in (49.9%), and 11 were (30.5%) unimicrobial. The causative bacteria were predominantly Gram-negative bacilli. The overall picture was as Staphylococcus spp. (11), Acenitobacter spp. (15), Corynebacterium xerosis (03), Pseudomonas spp. (03), Klebsiella spp. (02), and Enterobacter (02).
Coexistent sepsis was present in 58 (44.6%) patients at the time of ventriculostomy insertion and 22 (17%) of these had a potential “open” source of infection in the form of a tracheostomy, pressure sore, or wound infection. The common infections were chest (24), urinary tract infections (20), wound infection (14), septicaemia (10), tracheostomy related (08), and bedsore related (04). Ventriculostomy catheters were not changed in 91 (70%) patients; the rest underwent sequential changes of their ventricular catheters. In 21 patients the catheter was changed once only, twice in 12 patients, and thrice in only 6 patients.
In 36 patients who developed EVD infection, the catheter that got infected was the first one in 18 patients, second in 15 patients, and third in the remaining 3 patients. In noninfected group, proportionately much fewer patients underwent any catheter changes. 13 out of 81 noninfected patients underwent catheter changes compared to 20 of the 36 infected patients.
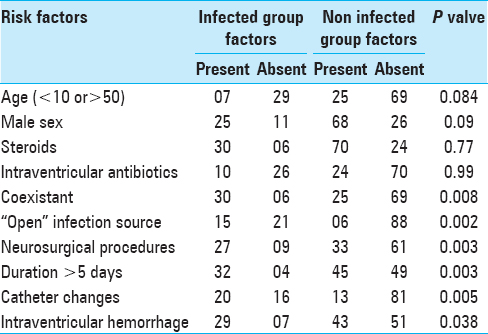
The total duration of catheter varied from 1 to 32 days. (Mean 11.5 days, median 4 days), the largest group was 58 patients having total catheter duration of 1–5 days. Only 3 patients had catheters for more than 30 days. Twenty-two patients had catheter for 6–10 days, 16 patients for 11–15 days, 18 patients for 16–20 days, 11 patients for 21–25 days, and 7 patients had catheter for 26–30 days. The day of onset of infection varied from 5 to 25 days, mean day of onset being 9.5 days. An additional neurosurgical procedure was performed in 40 patients, with preoperative EVD in 12 patients, intraoperative EVD insertion in 18 patients, and postoperatively insertion of ventriculostomy in the remaining 10 patients. Radiologically, evident IVH was seen in 78 (60%) cases, which was the cause of hydrocephalus in these patients. Many potential risk factors were examined for their relation to ventriculitis and are shown in
Coexistent sepsis was present in 30 of the 36 patients in the infected group compared to 25 of the 94 noninfected patients. Similarly, bedsores, tracheostomy, or wound infection were patients in 15 of the 36 infected patients compared to only 6 of the 94 noninfected patients. 27 of the 36 patients with EVD-associated infection had undergone another neurosurgical procedure compared to only 23 among 94 of the noninfected group, thus resulting in a significant associated.
The relationship of catheter changes to infection was extremely complex to describe; however, performance of the Chi-square test revealed significant association between catheter changes and infection as 20 of 36 patients in infected group had undergone catheter changes (16 patients had catheter changed once, 4 patients had changed twice) compared to 13 of the 94 noninfected patients (5 patients had single catheter change, 6 patients had catheter changed twice, and 2 patients had catheter changed thrice). However, prior ventriculostomy was not a significant factor in the univariate and multivariate analysis. The presence of IVH was also well correlated with infection in our patients, with 29 of 36 infected patients (74%) having IVH compared to only 43 of the 94 (46%) noninfected. The total duration of ventricular catheters had a significant correlation with infection. The mean total duration of ventriculostomy in the infected group was 20.5 days, (mean duration prior to infection was 7.5 days) compared to 4.4 days in the noninfected group. A life table analysis was performed taking into account the day of infection which revealed a 6% infection rate by the day 5 which became 13% by days 10, 17% by day 15, 21% by day 20, 29% by day 25, 36% by day 30, and 46% by days above 30.
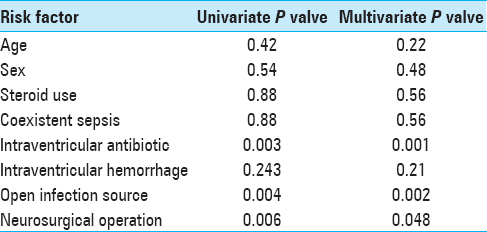
A univariate analysis was also undertaken to define the relative strengths of the contributory factors, which revealed that coexistent sepsis. “Open” infection source, IVH, and another neurosurgical operation were all contributory factors [
Besides infection which was the most common complication of the procedure, the second common complication seen was catheter blockage in 34 patients (26.15%). Generally, the blockage was minor in nature observed in 21 patients, and simple manipulation would clear the block. On the other hand, 13 patients with more severe blockage required a change of catheter for only underwent a change of catheter for blockage while as in 20 patients catheters were changed for infections.
Two patients (1.53%) during the procedure developed small intracerebral hematoma due to direct vascular trauma, thus presenting with an incidence of 1.53%. Both the patients were asymptomatic, and hematomas resolved completely with conservative management. In our study seizures were noticed in 18 patients (13.8%), mostly in children (12 patients).
Evaluation of outcome of patients revealed that there was an overall mortality of 61 (46.9%) patients both in the acute phase and later. 33 of the 39 patients having GCS 3–5 at the time of EVD insertion expired, as against 20 of the 51 patients in GCS 6–8. Patients in GCS 9–12 had even better outcome, with 8 of the 35 patients in this group expiring. EVD infection was mostly prevalent in patients with GCS 8 or below, with 33 of the 90 infected patients being in this group. The percentage of patients in a particular group who had infection were 51.2% in the GCS 3–5 group, 25.4% in the GCS 6–8 group, 8.5% in the GCS 9–13 group, and none in the GCS 14–15 group. Ventriculitis as the cause of death was similarly dominant in the lower GCS categories [
DISCUSSION
EVD is the procedure of choice for the treatment of acute hydrocephalus and increased ICP in patients of SAH and intracerebral hemorrhage with hydrocephalus and its sequelae. Despite the risk of infection and the other known complications associated with the procedure, the benefits for outweigh the risks involved. Applying EVD has positive results and influence the prognosis, and early and late complications of SAH. Acute hydrocephalus is defined as clinically and radiographically demonstrated ventricular dilatation that developed within 2 weeks of the onset of SAH. Acute hydrocephalus is a well-documented complication of SAH. Several studies have implicated a significantly increased risk of rebleeding in patients with EVD, compared with patients without extraventricular drainage. Abrupt lowering of ICP could lead to rebleeding due to decreased transmural pressure or removal of clot sealing the previously ruptured aneurysm. However, a variety of parameters affect the rebleeding rate, such as the timing of surgery, the timing and duration of drainage, the size of the aneurysm, as well as severity of initial hemorrhage.[
The 27.6% rate of infection in our patients fall within the 0–40% range quoted in other studies.[
The high rate of infection is also attributed to the higher percentage of our patients who required a ventricular catheter for a longer duration compared to other studies.[
The high proportion of skin contaminants and commensal organisms in our study points of the importance of colonization of the catheter assembly. Others[
Age had no influence on infection rate, and neither did the use of corticosteroids. Both these findings were contrary to expected results but could be due to relatively small numbers in subcategories, resulting but could be due to relatively small numbers in subcategories, resulting in a low statistical power. There was a relationship between IVH and infection as has been reported in at least two other prospective studies.[
Patients who had another neurosurgical procedure performed were at significantly higher risk, along with immunosuppression, which accompanies trauma and operative procedure, may also have a predisposing effect. This phenomenon has also been reported.[
The essence of this study is the unequivocal demonstration of a significant relationship between total duration of ventricular catheters and ventriculitis. This was clear even from the difference in mean catheter durations among infected and noninfected groups: 7.5 days (corrected) in infected group versus 4.4 days in noninfected group. Survival analysis revealed a linear rise in infection rates up to 5 days, beyond which there was an almost logarithmic rise in infection rates until 14 days, by which time infection was nearly universal. By definition, the survival analysis does not take into account the further duration of catheters once infection has taken place. On review of literature, we found this to be a shortcoming in at least 3 studies,[
Overall, EVD may seem to incur a sizable risk of infection. However, as our outcome data shows, this infection was monthly prevalent in the low GCS group. Even in the low GCS group, we were able to save 3 of 39 patients with GCS 3–5. Patients with better GCSs had even better outcomes, and with far less risk of infective complications. In poor group having gross IVH and ventriculomegaly, there are very few alternatives to performing a ventriculostomy despite the high rate of infection.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Albright L, Reigel DH. Management of hydrocephalus secondary to posterior fossa tumors. J Neurosurg. 1977. 46: 52-5
2. Ammirati M, Raimondi AJ. Cerebrospinal fluid shunt infections in children. A study on the relationship between the etiology of hydrocephalus, age at the time of shunt placement, and infection rate. Childs Nerv Syst. 1987. 3: 106-9
3. Bering EA. A simplified apparatus for constant ventricular drainage. J Neurosurg. 1951. 8: 450-2
4. Blomstedt GC. Results of trimethoprim-sulfamethoxazole prophylaxis in ventriculostomy and shunting procedures. A double-blind randomized trial. J Neurosurg. 1985. 62: 694-7
5. Bogdahn U, Lau W, Hassel W, Gunreben G, Mertens HG, Brawanski A. Continuous-pressure controlled, external ventricular drainage for treatment of acute hydrocephalus – Evaluation of risk factors. Neurosurgery. 1992. 31: 898-903
6. Boulard G, Ravussin P, Guérin J. A new way to monitor external ventricular drainage. Neurosurgery. 1992. 30: 636-8
7. Chan KH, Mann KS. Prolonged therapeutic external ventricular drainage: A prospective study. Neurosurgery. 1988. 23: 436-8
8. Chaparro MJ, Pritz MB, Yonemura KS. Broviac ventriculostomy for long-term external ventricular drainage. Pediatr Neurosurg 1991. 1992. 17: 208-12
9. Clark WC, Muhlbauer MS, Lowrey R, Hartman M, Ray MW, Watridge CB. Complications of intracranial pressure monitoring in trauma patients. Neurosurgery. 1989. 25: 20-4
10. Dias MS, Albright AL. Management of hydrocephalus complicating childhood posterior fossa tumors. Pediatr Neurosci. 1989. 15: 283-9
11. Donauer E, Drumm G, Moringlane J, Ostertag C, Kivelitz R. Intrathecal administration of netilmicin in gentamicin-resistant ventriculitis. Acta Neurochir (Wien). 1987. 86: 83-8
12. Fishman RA.editors. Cerebrospinal Fluid Findings in Diseases of the Nervous System. Philadelphia: WB Saunders; 1980. p. 168-326
13. Fountas KN, Kapsalaki EZ, Machinis T, Karampelas I, Smisson HF, Robinson JS. Review of the literature regarding the relationship of rebleeding and external ventricular drainage in patients with subarachnoid hemorrhage of aneurysmal origin. Neurosurg Rev. 2006. 29: 14-8
14. Friedman WA, Vries JK. Percutaneous tunnel ventriculostomy. Summary of 100 procedures. J Neurosurg. 1980. 53: 662-5
15. Gerner-Smidt P, Stenager E, Kock-Jensen C. Treatment of ventriculostomy-related infections. Acta Neurochir (Wien). 1988. 91: 47-9
16. Holloway KL, Barnes T, Choi S, Bullock R, Marshall LF, Eisenberg HM. Ventriculostomy infections: The effect of monitoring duration and catheter exchange in 584 patients. J Neurosurg. 1996. 85: 419-24
17. Khanna RK, Rosenblum ML, Rock JP, Malik GM. Prolonged external ventricular drainage with percutaneous long-tunnel ventriculostomies. J Neurosurg. 1995. 83: 791-4
18. Kim DK, Uttley D, Bell BA, Marsh HT, Moore AJ. Comparison of rates of infection of two methods of emergency ventricular drainage. J Neurol Neurosurg Psychiatry. 1995. 58: 444-6
19. Kusske JA, Turner PT, Ojemann GA, Harris AB. Ventriculostomy for the treatment of acute hydrocephalus following subarachnoid hemorrhage. J Neurosurg. 1973. 38: 591-5
20. Lundberg N, Troupp H, Lorin H. Continuous recording of the ventricular-fluid pressure in patients with severe acute traumatic brain injury. A preliminary report. J Neurosurg. 1965. 22: 581-90
21. Mayhall CG, Archer NH, Lamb VA, Spadora AC, Baggett JW, Ward JD. Ventriculostomy-related infections. A prospective epidemiologic study. N Engl J Med. 1984. 310: 553-9
22. McLaurin RL. Disadvantages of the preoperative shunt in posterior fossa tumors. Clin Neurosurg. 1983. 30: 286-92
23. Miller JD, Hoff JT, Betz AL.editors. Measuring ICP in patients. Its value now and in the future?. Intracranial Pressure VII. Berlin, Heidelberg, New York, London, Paris, Tokyo, Hong Kong: Springer; 1989. p. 5-15
24. Narayan RK, Kishore PR, Becker DP, Ward JD, Enas GG, Greenberg RP. Intracranial pressure: To monitor or not to monitor?. A review of our experience with severe head injury. J Neurosurg. 1982. 56: 650-9
25. Ohrstrom IM, Castilli MA. Transorbital ventricular puncture for emergency ventricular decompression. J Neurosurg. 1981. 54: 273-4
26. Pampus F. Technic of ventricular drainage. Zentralbl Neurochir. 1953. 13: 219-23
27. Paramore CG, Turner DA. Relative risks of ventriculostomy infection and morbidity. Acta Neurochir (Wien). 1994. 127: 79-84
28. Rosner MJ, Becker DP. ICP monitoring: Complications and associated factors. Clin Neurosurg. 1976. 23: 494-519
29. Shapiro S, Boaz J, Kleiman M, Kalsbeck J, Mealey J. Origin of organisms infecting ventricular shunts. Neurosurgery. 1988. 22: 868-72
30. Smith RW, Alksne JF. Infections complicating the use of external ventriculostomy. J Neurosurg. 1976. 44: 567-70
31. Stenager E, Gerner-Smidt P, Kock-Jensen C. Ventriculostomy-related infections – An epidemiological study. Acta Neurochir (Wien). 1986. 83: 20-3
32. Sundbärg G, Nordström CH, Söderström S. Complications due to prolonged ventricular fluid pressure recording. Br J Neurosurg. 1988. 2: 485-95
33. Winfield JA, Rosenthal P, Kanter RK, Casella G. Duration of intracranial pressure monitoring does not predict daily risk of infectious complications. Neurosurgery. 1993. 33: 424-30
34. Wyler AR, Kelly WA. Use of antibiotics with external ventriculostomies. J Neurosurg. 1972. 37: 185-7