- Department of Neurosurgery, Cristo Redentor Hospital,
- Graduate Program in Surgical Sciences, Federal University of Rio Grande do Sul - Brazil, Porto Alegre,
- Department of Neurosurgery, Federal University of Pelotas, Pelotas, RS, Brazil.
Correspondence Address:
Otávio Garcia Martins
Graduate Program in Surgical Sciences, Federal University of Rio Grande do Sul - Brazil, Porto Alegre,
DOI:10.25259/SNI_203_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Guilherme Finger, Otávio Garcia Martins, William Mazzucco Nesi, Mateus Carvalho Casarin, Leandro Pelegrini de Almeida, Felipe Lourenzon Schiavo, Samir Cezimbra dos Santos, Marco Antonio Stefani. Ruptured aneurysm in the posterior communicating segment of carotid artery presenting with contralateral oculomotor nerve palsy. 13-Sep-2019;10:177
How to cite this URL: Guilherme Finger, Otávio Garcia Martins, William Mazzucco Nesi, Mateus Carvalho Casarin, Leandro Pelegrini de Almeida, Felipe Lourenzon Schiavo, Samir Cezimbra dos Santos, Marco Antonio Stefani. Ruptured aneurysm in the posterior communicating segment of carotid artery presenting with contralateral oculomotor nerve palsy. 13-Sep-2019;10:177. Available from: http://surgicalneurologyint.com/surgicalint-articles/9642/
Abstract
Background: Brain aneurysms are mostly discovered during the investigation of subarachnoid hemorrhage (SAH). Some patients present neurological signs that may suggest the aneurysm’s topography, and the oculomotor nerve palsy (ONP) of the same side of the aneurysm is the most common sign. Only one case report of contralateral palsy was previously described in the medical literature.
Case Description: Authors describe a patient who presented a classic manifestation of SAH associated with complete ONP, whose vascular investigation demonstrated a brain aneurysm located in the contralateral intracranial carotid. The patient was surgically treated with great neurologic outcome, and late angiography did not evidence other vascular abnormalities.
Conclusion: The ipsilateral ONP is a common sign found in posterior communicating artery aneurysms; however, such aneurysm can have different presentations due to the elevation of intracranial pressure, and, in rarer cases, the ONP cannot be operated as a localizing sign.
Keywords: Brain aneurysm, Cranial mononeuropathy, Oculomotor nerve disease, Ruptured intracranial aneurysm, Subarachnoid hemorrhage
INTRODUCTION
Subarachnoid hemorrhage (SAH) is a condition characterized by the presence of blood in the subarachnoid space, whose origin may be secondary to arterial dissection, trauma, and venous or aneurysm rupture.[
Oculomotor nerve palsy (ONP) is a classic localizing sign of aneurysms situated along its course, being the posterior communicant artery (PComA) or communicant segment of internal carotid artery (ICA), the main locations of aneurysms associated with ONP at the same side of the aneurysm.[
This case report describes the third case of ONP associated with contralateral ICA-PComA junction aneurysm,[
CASE REPORT
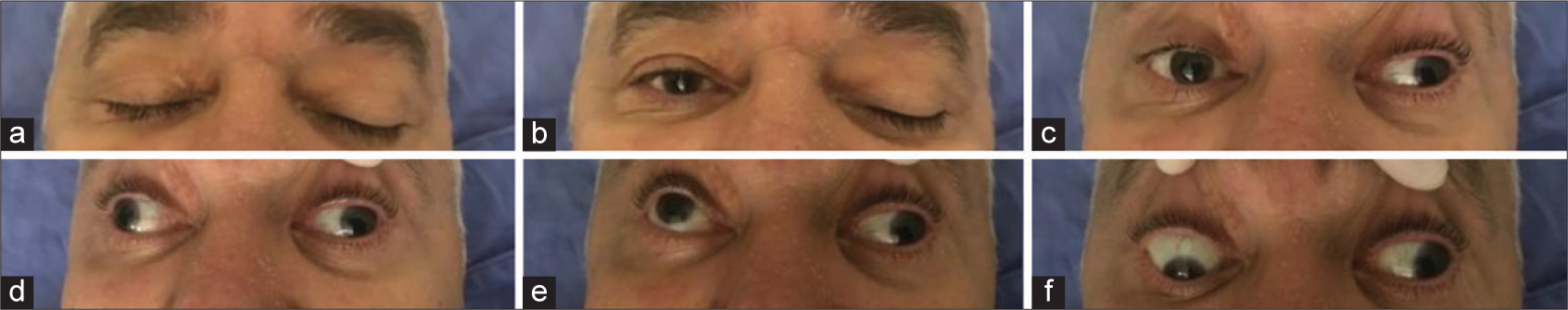
A 60-year-old male, with no previous medical comorbidity, woke up during the night with a severe sudden headache. The patient tried to get up from the bed when he lost consciousness. The patient could not determine how long he persisted unconscious, but when he recovered mental functions, he called for medical support (patient lives alone). According to the patient, since he recovered consciousness, he persisted with a painful headache noticing that he could not open his left eyelid. The urgent medical assistance team promptly forwarded the patient to a tertiary hospital. At emergency admission, the patient was conscious, interacting adequately with the physicians, mobilizing all members without motor or sensitive deficits, but presented a marked neck stiffness and positive Brudzinski’s sign. The patient presented a complete left eyelid ptosis, with divergent strabismus due to a lateral rotation of the left eyeball and anisocoria with the left pupil mydriasis. Ocular extrinsic movement of the right eyeball was preserved; however, the superior, inferior, and medial movement of the left eyeball was compromised [
Figure 1:
Ocular extrinsic examination at hospital admission demonstrating complete left oculomotor nerve palsy. (a) Both eyelids closed. (b) Complete ptosis of the left eyelid. (c) The patient looking forward, demonstrating a left rotation of the left eyeball due to abducens nerve function not balanced by the oculomotor nerve stimuli over the medial rectus muscle. (d) The patient looking to the right, without medial movement of the left eyeball. (e) The patient looking upward without superior vertical movement of the left eyeball. (f) The patient looking downward without inferior vertical movement of the left eyeball.
DISCUSSION
Aneurysmal SAH usually presents unspecific symptoms such as severe headache, vomiting, and photophobia. These symptoms, despite its importance to suspicion of SAH, do not allow the topographic diagnosis of the aneurysm. However, the presence of certain infrequent specific signs can suggest the aneurysm position, operating as localizing signs.[
The oculomotor nerve emerges from the interpeduncular fossa in the midbrain, medially to the cerebral peduncles. At its point of emergence of the brainstem, it is closely related to the posterior cerebral artery (PCA) superiorly and superior cerebellar artery (SCA) inferiorly. Furthermore, the nerve gets its vascular supply from the basilar system at this point. It passes inferomedial to the uncus and inferolateral to the PComA to enter the roof of the cavernous sinus (CS) through the oculomotor trigone. Up to this point, the nerve can be affected by several pathological processes such as tumors, clots in the interpeduncular cistern, and direct compression by aneurysms of the underlying arteries.[
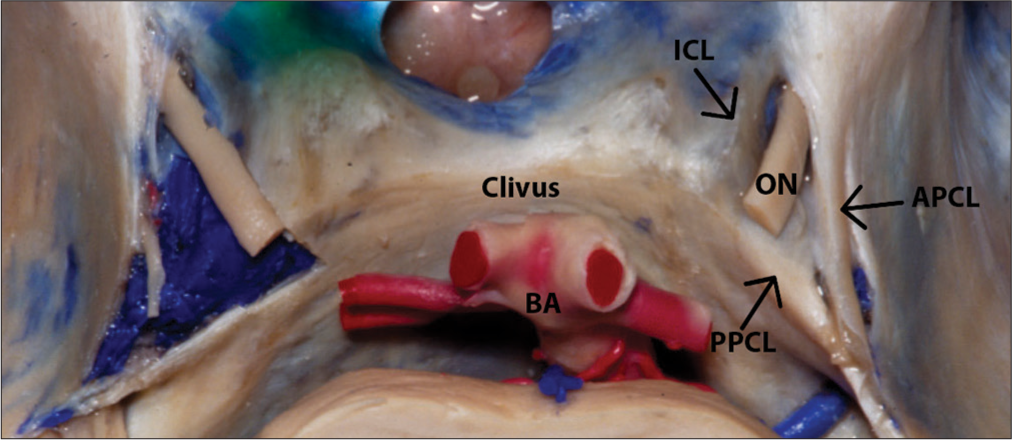
The cavernous segment of the oculomotor nerve runs through the lateral wall of the CS superiorly. The nerve enters on the CS passing through the oculomotor triangle, which is delimited by the anterior petroclinoid ligament, the posterior petroclinoid ligament, and the interclinoid ligament [
Figure 3:
Entry of the oculomotor nerve into the cavernous sinus through the oculomotor triangle, which is a potential compression point. Structures of the oculomotor triangle are shown. ON - Oculomotor nerve, BA - Basilar artery, APCL - Anterior petroclinoid ligament, PPCL - Posterior petroclinoid ligament, ICL - Interclinoid ligament. Courtesy of the Rhoton Collection, American Association of Neurological Surgeons/Neurosurgical Research and Education Foundation.
The ICA-PComA junction is the most common location of aneurysm that courses with ipsilateral ONP, with a prevalence of 34%–56% among ruptured cases.[
In the aneurysm scenario, the main mechanism of ipsilateral ONP is related to the compression of the nerve fibers by the aneurysm itself, increased ICP with or without brain herniation, compression within the perimesencephalic cisterns by focal blood clot, or chemical lesion by the blood metabolites in subarachnoid space.[
Especially in this case, authors hypothesize that elevation of ICP may be more strongly pointed as the etiological factor of the contralateral ONP due to the establishment of the deficit in the ictus of the event. A sudden elevation of ICP may compress the oculomotor nerve at its vulnerable points into the CS roof or in the oculomotor trigone.[
Somagawa et al. reported a similar case of an acute SAH of the ICA-PComA junction with contralateral ONP.[
Another case reported was a patient of Sun et al., who presented a PComA-ICA junction aneurysm that was treated with clipping.[
In our case, the definitive treatment of the aneurysm with clipping was not associated with early improvement; however, at 6 months of follow-up, he had partially recovered the nerve’s functions. The recovery from nerve palsy depends on the time between the onset of symptoms and surgery. The earlier the operation is done, the higher is the chance of nerve function recovery.[
This case may demonstrate that ONP may behave as a nontopographic sign in cerebral aneurysmal disease, and advanced neuroimaging is required to determine the location of aneurysms in patients with SAH.
CONCLUSION
Contralateral ONP is an extremely rare symptom of ICA-PComA aneurysm with only two cases reported, including ours. The pathophysiology of this phenomenon may depend on several factors such as intracranial hypertension, the presence of clots in the subarachnoid space, or the toxic effect of blood metabolites. In cases of late contralateral ONP, other events such as vasospasm and rebleeding may also be associated.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understand that his names and initials will not be published and due efforts will be made to conceal his identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Broderick JP, Brott T, Tomsick T, Miller R, Huster G. Intracerebral hemorrhage more than twice as common as subarachnoid hemorrhage. J Neurosurg. 1993. 78: 188-91
2. Cianfoni A, Pravatà E, De Blasi R, Tschuor CS, Bonaldi G. Clinical presentation of cerebral aneurysms. Eur J Radiol. 2013. 82: 1618-22
3. Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage. N Engl J Med. 2000. 342: 29-36
4. Fujiwara S, Fujii K, Nishio S, Matsushima T, Fukui M. Oculomotor nerve palsy in patients with cerebral aneurysms. Neurosurg Rev. 1989. 12: 123-32
5. Hop JW, Rinkel GJ, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: A systematic review. Stroke. 1997. 28: 660-4
6. Hoya K, Kirino T. Traumatic trochlear nerve palsy following minor occipital impact–four case reports. Neurol Med Chir (Tokyo). 2000. 40: 358-60
7. Kang SD. Ruptured anterior communicating artery aneurysm causing bilateral oculomotor nerve palsy: A case report. J Korean Med Sci. 2007. 22: 173-6
8. Keane JR. Third nerve palsy: Analysis of 1400 personally-examined inpatients. Can J Neurol Sci. 2010. 37: 662-70
9. Laun A, Tonn JC. Cranial nerve lesions following subarachnoid hemorrhage and aneurysm of the circle of willis. Neurosurg Rev. 1988. 11: 137-41
10. Linskey ME, Sekhar LN, Hirsch W, Yonas H, Horton JA. Aneurysms of the intracavernous carotid artery: Clinical presentation, radiographic features, and pathogenesis. Neurosurgery. 1990. 26: 71-9
11. Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: A meta-analysis. Lancet Neurol. 2009. 8: 635-42
12. Park HK, Rha HK, Lee KJ, Chough CK, Joo W. Microsurgical anatomy of the oculomotor nerve. Clin Anat. 2017. 30: 21-31
13. Plum F, Posner JB, Davis F.editors. The Diagnosis of Stupor and Coma. Philadelphia, PA: F. A. Davis Co.; 1980. p.
14. Shukla DP, Bhat DI, Devi BI. Anterior communicating artery aneurysm presenting with vision loss. J Neurosci Rural Pract. 2013. 4: 305-7
15. Somagawa C, Fukuda Y, Yoshimura S, Satoh K, Hiu T, Ono T. A rare case of ruptured internal carotid-posterior communicating artery aneurysm associated with contralateral delayed oculomotor nerve palsy. No Shinkei Geka. 2017. 45: 629-35
16. Soni SR. Aneurysms of the posterior communicating artery and oculomotor paresis. J Neurol Neurosurg Psychiatry. 1974. 37: 475-84
17. Sun Y, Li G, Hou K, Gao X, Zhu X. A case of delayed contralateral oculomotor nerve palsy after microsurgical clipping of a posterior communicating artery aneurysm. Int J Clin Exp Med. 2016. 9: 12964-6
18. van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007. 369: 306-18
19. Yanaka K, Matsumaru Y, Mashiko R, Hyodo A, Sugimoto K, Nose T. Small unruptured cerebral aneurysms presenting with oculomotor nerve palsy. Neurosurgery. 2003. 52: 553-7