- Department of Neurosurgery, Charing Cross Hospital, Imperial College of London, London
- Department of Neurosurgery, Aberdeen Royal Infirmary, NHS Grampian, Edinburgh, Scotland, UK
- Department of Pathology, NHS Lothian, Western General Hospital, Edinburgh, Scotland, UK
Correspondence Address:
Giulio Anichini
Department of Neurosurgery, Aberdeen Royal Infirmary, NHS Grampian, Edinburgh, Scotland, UK
DOI:10.4103/2152-7806.183520
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Anichini G, Iqbal M, Rafiq NM, Ironside JW, Kamel M. Sacrificing the superior petrosal vein during microvascular decompression. Is it safe? Learning the hard way. Case report and review of literature. Surg Neurol Int 03-Jun-2016;7:
How to cite this URL: Anichini G, Iqbal M, Rafiq NM, Ironside JW, Kamel M. Sacrificing the superior petrosal vein during microvascular decompression. Is it safe? Learning the hard way. Case report and review of literature. Surg Neurol Int 03-Jun-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/sacrificing-the-superior-petrosal-vein-during-microvascular-decompression-is-it-safe-learning-the-hard-way-case-report-and-review-of-literature/
Abstract
Background:Venous infarction as a complication of microvascular decompression (MVD) is a recognized but extremely rare occurrence in an otherwise standard neurosurgical procedure. Sacrificing one or more veins is considered safe by majority of experienced surgeons and authors. However, in the recent years, there has been growing debate about the management of venous trigeminal compression and/or superior petrosal complex (separation vs. coagulation and cutting of the vein), with few papers describing mild to severe complications related to venous sacrifice.
Case Description:We report our dramatic experience during re-exploration for MVD on a male who developed massive cerebellar, brainstem, and brain infarction. Extensive analysis of surgical planning and literature debate about this topic is also reported.
Conclusion:Despite rare, venous infarction after venous sacrifice in MVD is possible and can have catastrophic consequences. We would advise: (1) To try preserving the vein anytime this is possible, especially if it is large in size; (2) if it is decided to sacrifice the vein temporary occlusion while observing changed in the neurophysiology might be safer; (3) when planning an MVD for suspected venous compression, possible alternative forms of treatment should also be considered.
Keywords: Dural arteriovenous fistula, microvascular decompression, petrosal vein, trigeminal neuralgia, venous infarction
INTRODUCTION
Microvascular decompression (MVD) for trigeminal neuralgia is a commonly practiced procedure in neurosurgery, and several large series have been reported so far.[
We describe a case of a patient who developed a massive nervous tissue infarction during re-exploration of the trigeminal nerve for MVD. Critical analysis of the circumstances and literature review is also presented.
CASE ILLUSTRATION
A 55-year-old male presented with classical symptoms of the left trigeminal neuralgia. He reported several daily episodes of intense facial pain on the left side of the face, occurring around 20–30 times/day. Pain episodes were described as sharp, with sudden onset, lasting few seconds and completely unpredictable. The pain involved mainly V2 and V3 branches but occasionally also V1 branch was reported. At the time of neurosurgical assessment, the pain was going on from years, and the patient had already tried medications, unsuccessfully.
Magnetic resonance imaging (MRI) of the brain with time of flight, arterial, and venous sequences was performed to identify possible microvascular compression. This was actually seen at the level of the left trigeminal nerve, at its exit from the pons. A large venous vessel tributary of the petrous vein complex was found close to the nerve, and it was suspected to be responsible for the pain [Figure
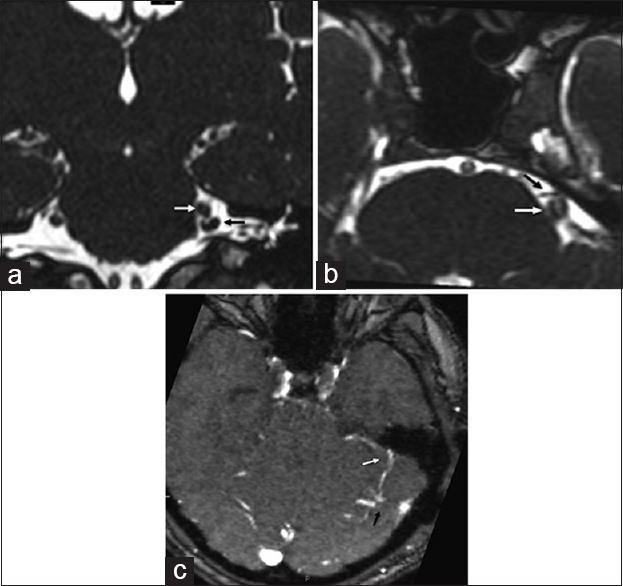
Figure 1
Magnetic resonance imaging scan, time of flight sequences; (a) coronal section showing the venous loop (black arrow) around the exit of the V nerve (white arrow); (b) axial section, time of flight sequences, showing possible anterior arterial conflict; (c) magnetic resonance venography showing the vein anatomy of the patient: The compressing vein is draining into the superior petrosal complex (white arrow); a possible vascular abnormality (black arrow) is seen into the right cerebellar hemisphere
The patient was therefore scheduled for MVD. This was carried out through a small (2, 5 cm in size) retro-sigmoid craniotomy and cerebellopontine angle exploration, under continuous neurophysiological monitoring (V, VII, and brainstem auditory evoked response [BAER]). After opening the cisterna magna and performing cerebro-spinal fluid release, cerebellum relaxation was obtained, and the left cerebellopontine angle in its superior compartment was exposed. The superior petrosal complex was partially covering the surgical trajectory. However, by using a different angle and performing gentle cerebellar retraction, it was possible to preserve it while still getting a good visual exposure of the V cranial nerve complex. The trigeminal nerve was markedly compressed by a large vein – probably the cerebellopontine vein itself, which was draining into the superior petrosal complex [
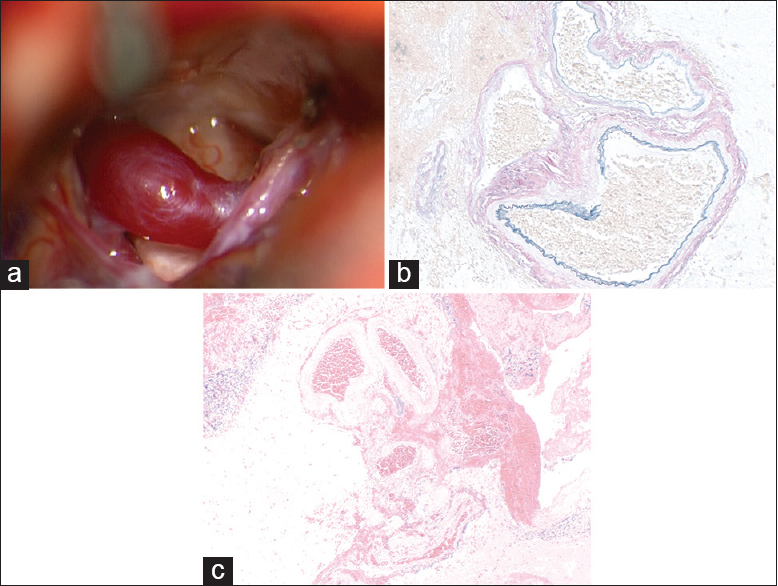
Figure 2
(a) Intra-operative view. Large, dilated vein is markedly compressing the trigeminal nerve; the vein appears to drain into the superior petrosal complex, which is partially seen on the right side of the surgical field. (b) Abnormally thickened and dilated vessels occurring in clusters in the cerebellar cortex and subarachnoid space, (H and Eosin, ×40); (c) these abnomal vessels show irregular fibrosis (red) and elastosis (black) on an Elastica-van Gieson stain, in keeping with arterialization of venous channels (×100)
Postoperative course was uneventful and without complications. Patient experienced immediate relief from the pain and was discharged after 4 days. However, after 10 days from the operation, the patient reported that the pain was coming back again. There was still a consistent clinical improvement; since the pain was now located just at the level of the maxillary branch of the trigeminal nerve (V2) while the pain on the remaining branches had disappeared. Despite this, the pain on V2 was described as the same as before. Medical treatment with carbamazepine was attempted again, but unsuccessfully, and the patient asked for other treatment options. A new MRI scan was performed showing good separation of the vein from the nerve, but still possible arterial conflict medial to the nerve. Radiofrequency, glycerol injection and surgical re-exploration were all discussed with the patient, who was not keen on trying conservative management or alternative treatments other than surgery. The case was re-discussed on the weekly neurosurgical department meeting and consultation with other experienced surgeons in the country was taken. An arterial conflict and maybe compression by the Teflon itself were suspected, and the consensus was to re-explore the nerve looking for any missed arterial conflict.
Re-do surgery was then scheduled. Through the same retrosigmoid approach, cisterna magna was opened, satisfactory cerebellar relaxation obtained, and cerebellopontine angle exposed. No significant cerebellar retraction was performed. To get proper exposure of the trigeminal nerve, this time the superior petrosal venous complex was coagulated and cut at its entrance into the superior petrosal sinus. Excellent exposure of the trigeminal nerve was then obtained: Previously placed Teflon separating the vein from the nerve was seen and partially removed. However, after about 10 min from the coagulation of the superior petrosal vein, an anesthetist advised about quite abrupt change in vital parameters: Tachycardia and high blood pressure were noted. At the same time, the surgeon noticed progressive cerebellar swelling and the cerebellopontine angle became quickly inaccessible, despite attempting cerebellar retraction. Cerebellar tissue started to swell beyond the craniotomy margins. Venous infarction was immediately suspected. Diffuse oozing and low-pressure bleeding was noted on the cerebellar surface. The craniotomy was enlarged medially and partial lateral cerebellar resection was also performed. A large clot coming from the cerebellar tissue was evacuated. Some abnormal vessels were found in the context of the cerebellar tissue, thus venous angioma/cerebellar arteriovenous malformation (AVM) was suspected, and samples were sent for pathology analysis, which confirmed the same [Figure
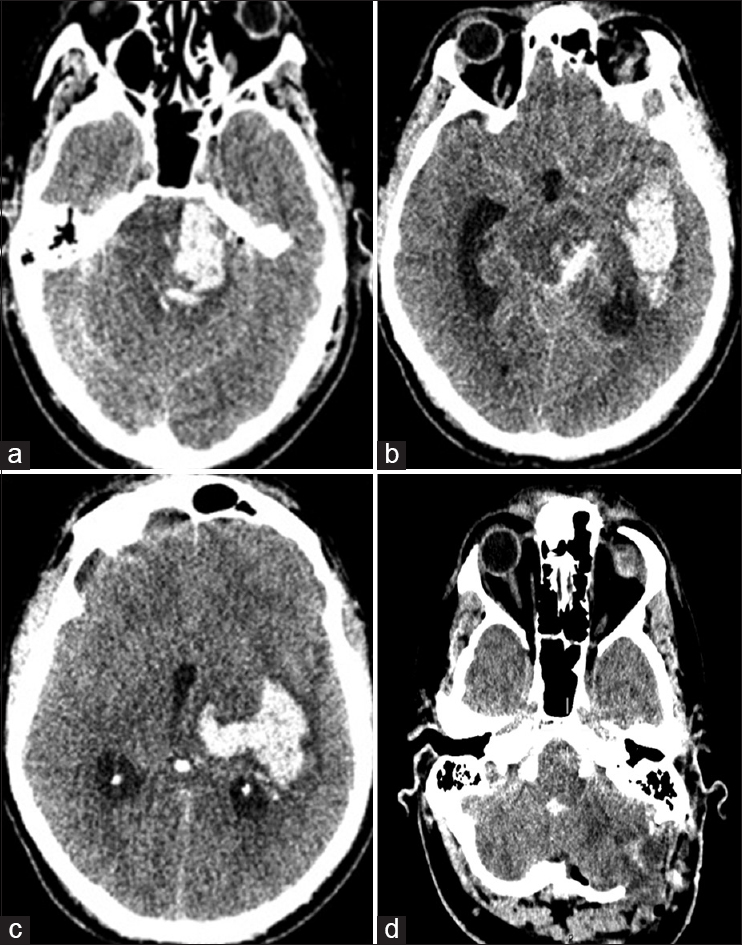
Figure 3
Computed tomography head done immediately after the second attempt of microvascular decompression, showing dramatic pontine (a), midbrain and basal temporal (b), posterior thalamus and mesial temporal lobe (c) infarction; craniectomy is noted at the level of the posterior cranial fossa (PCF) together with partial cerebellar lobectomy and clot removal (d)
DISCUSSION
MVD is a common procedure in neurosurgery, and large series have been reported so far.[
It is quite debated topic what to do in case of venous compression of the trigeminal nerve. Experienced surgeons advocated that vein responsible for the nerve compression can be taken without significant complications,[
The superior petrosal vein it is usually a major venous supply of the posterior cranial fossa. It is located in close proximity to the trigeminal nerve, below the tentorial edge, and it runs anteriorly and laterally from the upper portion of the cerebellopontine angle toward the petrous bone, into the superior petrosal sinus. In a recent anatomical report by Matsushima et al., four major vein groups have been described to be tributaries of the superior petrosal vein: (1) Petrosal group, draining the fourth ventricle, the lateral medulla, the cerebellopontine fissure, the petrosal cerebellar surface; (2) posterior mesencephalic group, draining the area facing the cerebello-mesencephalic fissure; (3) the anterior mesencephalic group, draining the anterior-lateral portion of the midbrain and pons; (4) the tentorial group, draining the lateral portion of the cerebellar surface facing the tentorium.[
Our main questions are concerning what could have been done differently. First of all, in the very first operation, we were quite concerned about sacrificing the vein responsible for compression, given its large size. Some authors reported use of neurophysiologic monitoring to detect early brainstem changes during temporary venous occlusion. In one particular case, reduction of brainstem auditory evoked potentials after temporary obstruction of the superior petrosal vein during surgery was seen, thus allowing the authors to spare the vein and avoid possible brainstem infarction.[
To sum things up, we would advise to try preserving the vein anytime this is possible, especially if it is large in size. If it is decided to sacrifice the vein temporary occlusion while observing changed in the BAER may make the procedure safer. When planning an MVD for suspected venous compression, possible alternative treatment should be advised whenever serious concerns of possible venous infarction.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Akhaddar A, Gazzaz M, Elmostarchid B, Boucetta M. Trigeminal neuralgia and dural arteriovenous fistula in an edentulous man. Headache. 2010. 50: 861-2
2. Cannizzaro D, Rammos SK, Peschillo S, El-Nashar AM, Grande AW, Lanzino G. The lateral mesencephalic vein: Surgical anatomy and its role in the drainage of tentorial dural arteriovenous fistulae. World Neurosurg. 2016. 85: 163-8
3. Chen HJ, Lui CC. Peduncular hallucinosis following microvascular decompression for trigeminal neuralgia: Report of a case. J Formos Med Assoc. 1995. 94: 503-5
4. Cohen-Gadol AA. Microvascular decompression surgery for trigeminal neuralgia and hemifacial spasm: Naunces of the technique based on experiences with 100 patients and review of the literature. Clin Neurol Neurosurg. 2011. 113: 844-53
5. Dandy WE. Concerning the cause of trigeminal neuralgia. Am J Surg. 1934. 24: 447-55
6. Du R, Binder DK, Halbach V, Fischbein N, Barbaro NM. Trigeminal neuralgia in a patient with a dural arteriovenous fistula in Meckel's cave: Case report. Neurosurgery. 2003. 53: 216-21
7. Ferroli P, Acerbi F, Broggi M, Broggi G. Arteriovenous micromalformation of the trigeminal root: Intraoperative diagnosis with indocyanine green videoangiography: Case report. Neurosurgery. 2010. 67: onsE309-10
8. Gardner WJ. Concerning the mechanism of trigeminal neuralgia and hemifacial spasm. J Neurosurg. 1962. 19: 947-58
9. Greenberg MS.editors. Microvascular decompression for trigeminal neuralgia. From Handbook of Neurosurgery. Greemberg Graphics, Inc. Tampa, FL (US): Thieme Verlag; 2010. p. 560-1
10. Grigorian IU, Stepanian MA. Trigeminal neuralgia and tentorial dural arteriovenous malformation. Zh Vopr Neirokhir Im N N Burdenko. 2010. 1: 46-51
11. Jannetta P. Microsurgical approach to the trigeminal nerve for tic doloureaux. Prog Neurol Surg. 1976. 7: 180-200
12. Jannetta PJ. Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg. 1967. 26: 159-62
13. Koerbel A, Wolf SA, Kiss A. Peduncular hallucinosis after sacrifice of veins of the petrosal venous complex for trigeminal neuralgia. Acta Neurochir (Wien). 2007. 149: 831-2
14. Lu X, Qin X, Ni L, Chen J, Xu F. Tentorial dural arteriovenous fistula manifesting as contralateral trigeminal neuralgia: Resolution after transarterial onyx embolization. BMJ Case Rep 2013. 2013. p.
15. Lucas Cde P, Zabramski JM. Dural arteriovenous fistula of the transverse-sigmoid sinus causing trigeminal neuralgia. Acta Neurochir (Wien). 2007. 149: 1249-53
16. Masuoka J, Matsushima T, Hikita T, Inoue E. Cerebellar swelling after sacrifice of the superior petrosal vein during microvascular decompression for trigeminal neuralgia. J Clin Neurosci. 2009. 16: 1342-4
17. Matsushige T, Nakaoka M, Ohta K, Yahara K, Okamoto H, Kurisu K. Tentorial dural arteriovenous malformation manifesting as trigeminal neuralgia treated by stereotactic radiosurgery: A case report. Surg Neurol. 2006. 66: 519-23
18. Matsushima K, Matsushima T, Kuga Y, Kodama Y, Inoue K, Ohnishi H. Classification of the superior petrosal veins and sinus based on drainage pattern. Neurosurgery. 2014. 10: 357-67
19. Matsushima T, Huynh-Le P, Miyazono M. Trigeminal neuralgia caused by venous compression. Neurosurgery. 2004. 55: 334-7
20. McLaughlin MR, Jannetta JP, Subach BR, Clyde BL. Coagulation of petrosal vein for MVD. J Neurosurg. 1999. 90: 1148-
21. McLaughlin MR, Jannetta PJ, Clyde BL, Subach BR, Comey CH, Resnick DK. Microvascular decompression of cranial nerves: Lessons learned after 4400 operations. J Neurosurg. 1999. 90: 1-8
22. McMonagle B, Connor S, Gleeson M. Venous haemangioma of the mandibular division of the trigeminal nerve. J Laryngol Otol. 2011. 125: 649-50
23. Nagata K, Nikaido Y, Yuasa T, Fujioka M, Ida Y, Fujimoto K. Trigeminal neuralgia associated with venous angioma – Case report. Neurol Med Chir (Tokyo). 1995. 35: 310-3
24. Ott D, Bien S, Krasznai L. Embolization of a tentorial dural arterio-venous fistula presenting as atypical trigeminal neuralgia. Headache. 1993. 33: 503-8
25. Peterson AM, Williams RL, Fukui MB, Meltzer CC. Venous angioma adjacent to the root entry zone of the trigeminal nerve: Implications for management of trigeminal neuralgia. Neuroradiology. 2002. 44: 342-6
26. Piatt JH, Wilkins RH. Treatment of tic douloureux and hemifacial spasm by posterior fossa exploration: Therapeutic implications of various neurovascular relationships. Neurosurgery. 1984. 14: 462-71
27. Rahme R, Ali Y, Slaba S, Samaha E. Dural arteriovenous malformation: An unusual cause of trigeminal neuralgia. Acta Neurochir (Wien). 2007. 149: 937-41
28. Raveau V, Marsot-Dupuch K, Levy C. Symptomatic trigeminal neuralgia caused by venous angioma of the posterior fossa. Ann Radiol (Paris). 1992. 35: 85-8
29. Rizzo M, Bosch EP, Gross CE. Trigeminal sensory neuropathy due to dural external carotid cavernous sinus fistula. Neurology. 1982. 32: 89-91
30. Singh D, Jagetia A, Sinha S. Brain stem infarction: A complication of microvascular decompression for trigeminal neuralgia. Neurol India. 2006. 54: 325-6
31. Strauss C, Naraghi R, Bischoff B, Huk WJ, Romstöck J. Contralateral hearing loss as an effect of venous congestion at the ipsilateral inferior colliculus after microvascular decompression: Report of a case. J Neurol Neurosurg Psychiatry. 2000. 69: 679-82
32. Strauss C, Neu M, Bischoff B, Romstöck J. Clinical and neurophysiological observations after superior petrosal vein obstruction during surgery of the cerebellopontine angle: Case report. Neurosurgery. 2001. 48: 1157-9
33. Trebbastoni A, D’Antonio F, Biasiotta A, Fiorelli M, de Lena C. Diffusion tensor imaging (DTI) study of a trigeminal neuralgia due to large venous angioma. Neurol Sci. 2013. 34: 397-9
34. Tsukamoto H, Matsushima T, Fujiwara S, Fukui M. Peduncular hallucinosis following microvascular decompression for trigeminal neuralgia: Case report. Surg Neurol. 1993. 40: 31-4
35. Ward C, Corns R, Offa-Jones B, Cheserem JB, Hardwidge C. Cerebellar infarction following division of Dandy's vein in microvascular decompression for trigeminal neuralgia. J Neurol Surg. 2012. B73: A380-
36. Watanabe T, Igarashi T, Fukushima T, Yoshino A, Katayama Y. Anatomical variation of superior petrosal vein and its management during surgery for cerebellopontine angle meningiomas. Acta Neurochir (Wien). 2013. 155: 1871-8
37. Yamamoto T, Suzuki M, Esaki T, Nakao Y, Mori K. Trigeminal neuralgia caused by venous angioma: Case report. Neurol Med Chir (Tokyo). 2013. 53: 40-3
38. Zhong J, Li ST, Xu SQ, Wan L, Wang X. Management of petrosal veins during microvascular decompression for trigeminal neuralgia. Neurol Res. 2008. 30: 697-700
39. Zhong J, Zhu J, Sun H, Dou NN, Wang YN, Ying TT. Microvascular decompression surgery: Surgical principles and technical nuances based on 4000 cases. Neurol Res. 2014. 36: 882-93