- Department of Neurosurgery, Cerrahpasa Medical Faculty, Istanbul University, Istanbul, Turkey
- Clinical Biochemistry Laboratory, Haseki Training and Research Hospital, Istanbul, Turkey
- Clinical Biochemistry Laboratory, Ministry of Health, Haseki Training and Research Hospital, Istanbul, Turkey
- Department of Neurosurgery, Ministry of Health, Samatya Training and Research Hostpital, Istanbul, Turkey
- Department of Obstetrics and Gynecology, Bezm-i Alem Vakif University, Medical Faculty, Istanbul, Turkey
Correspondence Address:
Taner Tanriverdi
Department of Obstetrics and Gynecology, Bezm-i Alem Vakif University, Medical Faculty, Istanbul, Turkey
DOI:10.4103/sni.sni_405_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Taner Tanriverdi, Rahsan Kemerdere, Berrin B. Inal, Odhan Yuksel, Humeyra O. Emre, Merdin Ahmedov, Oguz Baran, Seda Ates. Serum endocan levels before and after surgery on low-grade gliomas. 14-Mar-2017;8:32
How to cite this URL: Taner Tanriverdi, Rahsan Kemerdere, Berrin B. Inal, Odhan Yuksel, Humeyra O. Emre, Merdin Ahmedov, Oguz Baran, Seda Ates. Serum endocan levels before and after surgery on low-grade gliomas. 14-Mar-2017;8:32. Available from: http://surgicalneurologyint.com/surgicalint_articles/serum-endocan-levels-before-and-after-surgery-on-low%e2%80%91grade-gliomas/
Abstract
Background:Endocan has been shown to be a marker for several cancers and may show degree of malignancy. The aim of this study is to assess serum levels of endocan before and after surgery on low-grade gliomas (LGGs).
Methods:Endocan was assayed by commercially available enzyme-linked immunosorbent assay (ELISA) kits in a total of 19 patients and 12 controls. Serial serum samples were obtained before and after surgery (1st day, 1st week, and 1st month of surgery). Control samples were collected from cord blood during cesarean section. The results were compared with control brain tissues.
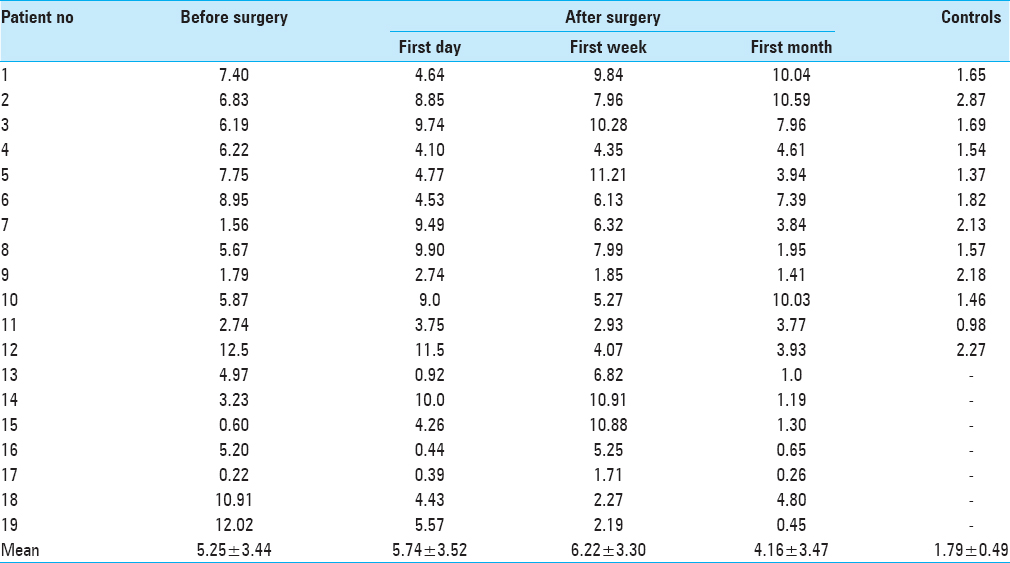
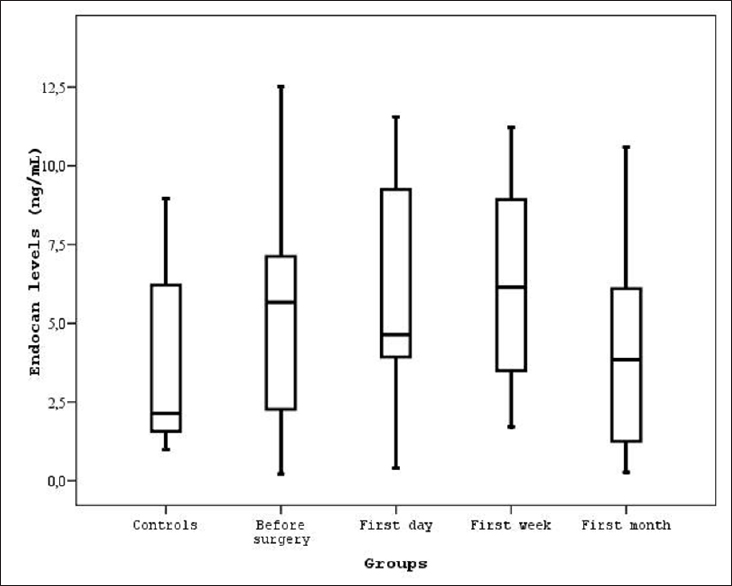
Results:Controls showed significantly lower serum endocan levels compared to before and after surgery (P
Conclusion:Endocan, a vital molecule for angiogenesis, is highly expressed before and after surgery in LGGs, but long-term data is needed. Furthermore, future studies should include high-grade gliomas to discuss whether endocan is associated with recurrence and response to treatment.
Keywords: Brain tumor, cancer, endocan, ESM-1, glioma, low-grade gliomas
INTRODUCTION
In neurosurgical practice, gliomas are commonly encountered brain tumors, and life expectancy in high-grade gliomas (HHGs) is very short. These most common brain tumors are re-classified as diffuse gliomas. According to this new classification, diffuse gliomas include World Health Organization (WHO) grade II and III astrocytic tumors, grade II and III oligodendrogliomas, grade IV glioblastomas, as well as the diffuse gliomas of childhood. Different from the previous classification, the astrocytomas, which have a more benign growth pattern, are now distinct from the diffuse gliomas.[
The rapidly expanding data related to endocan, previously known as endothelial cell-specific molecule-1 (ESM-1), has demonstrated that this soluble, freely circulating molecule in the blood could be used as a marker or could be a target not only in several tumors[
This molecule has been studied in serum or tumor tissues of patients or tumor lines of several tumors, however, there is little information regarding the levels of endocan in brain tumors. Thus, the current study is the first prospective study to show serum levels of endocan in patients with gliomas, LGGs here. A few studies related to endocan have been performed in pituitary adenomas;[
MATERIALS AND METHODS
Study population
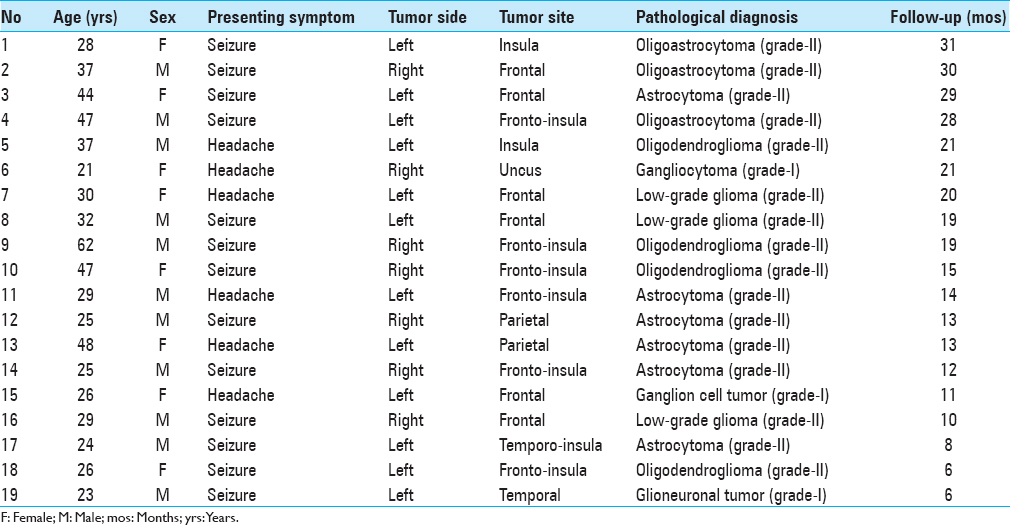
This study included 19 patients who were operated on for LGGs and gave permission and met our inclusion criteria for the study. All patients or next of kin were fully informed, and ethical approval for this study was obtained from the Human Investigations Committee at Istanbul University, Cerrahpasa Medical Faculty. We excluded patients who had any kind of chronic or acute infection, immunological and metabolic diseases, neoplastic disease of other organ systems, any cardiovascular diseases, and recent major surgical procedure at the time of tumoral tissue collection, in which endocan status might be affected. Furthermore, any patients who received radio or chemotherapy and had surgery of any kind of cerebral disorders before admission were also excluded. Controls consisted of 12 healthy women who had caesarian section and multiple births; those with major congenital anomalies were excluded.
Serum samples: Patients
Serum samples from 19 patients were obtained before surgery during the routine work-up and during routine follow-up visits after surgery. Before surgery, blood samples were obtained once, however, after surgery, serial blood samples were collected on the first day, first week, and first month. Thus, a total of four blood samples were collected from each patient. Each blood was centrifuged and serum samples were stored at −80°C until assayed for endocan.
Serum samples: Controls
The control group consisted of 12 women whose cord blood was collected at the time of elective cesarean delivery with spinal anesthesia. Umbilical cord blood samples were taken into vacutainer tubes and separated by centrifuging the samples at 500 g for 10 minutes. Serum samples again were stored at −80°C until assayed for endocan. Blood samples from the controls were obtained only once.
Assay of endocan
Endocan levels were measured with commercially available enzyme-linked immunosorbent assay (ELISA) kits (YH Biosearch Laboratory, Shanghai, China) according to the manufacturer's instructions. Briefly, after pipetting the serum samples into a 96-well microplate coated with a monoclonal antibody (also known as capture antibody) specific to the C-terminal of human endocan, incubation for 1 hour was performed. Endocan present within a sample was bound by the capture antibody. After washing the remaining unbound molecules, a biotinylated secondary monoclonal antibody (specific to the endocan N terminal) was added to the wells and allowed to incubate for 1 hour. Following the washing step, streptavidin-HRP (biotin-binding protein conjugated with polymers of horseradish peroxidase) was added and allowed to incubate in the dark for a further 30 minutes. Any remaining unbound material was again washed away. Chromogen solution was added and incubated in the dark for 10 minutes for converting the colorless solution into a blue solution, the intensity of which was proportional to the amount of endocan in the sample. Because of the acidic stop solution effect, the samples’ color turned yellow. Then, the colored reaction product was measured using an automated endocan reader at 450 nm. The unit of serum endocan was determined as ng/mL.
Statistical analysis
We used a commercially available statistical software package (SPSS version 14.0 Inc., Chicago, IL, USA) for all the statistical analyses. The mean ± standard deviations (±SD) were calculated for each parameter. For all comparisons, the nonparametric Mann–Whitney U test was used as a statistical method. Differences were considered statistically significant if the probability value was less than 0.05.
RESULTS
Demographics and serum levels of endocan in both patients and controls are shown in Tables
DISCUSSION
It is very difficult to discuss or compare our preliminary results obtained from the present study and with the current literature because there were no reports showing serum endocan levels in brain tumors. Serum endocan levels have been assessed in gastric cancer,[
It is not reasonable to discuss our preliminary results with the studies that evaluated serum levels of endocan in tumors of other organs including liver, bladder, and colon, etc. However; we can make some comparisons with the studies including immunohistochemical analysis of endocan in brain tumors. Our preliminary results are in line with the studies assessing serum levels of endocan in other cancers that serum endocan levels are significantly higher compared to the controls. Regarding immunohistochemical studies including HGGs and LGGs, our results are different in some respects. The present study showed that endocan can be expressed in LGGs too, which is in contrast to immunohistochemical study, which showed no endocan expression in LGGs.[
It is surprising that one of the highly vascularized organs, brain, does not contain endocan, although endocan is expressed from endothelium of vascularized organ.[
By depending solely on our limited number of patients, we cannot speculate that serum endocan levels can be used as a marker for recurrence in LGGs because we did not observe serum at the long-term follow-up (last visit) after surgery. However, we underline that endocan can be expressed in LGGs and can be detected in serum. Thus, in the future, serum endocan levels should be measured at the long-term follow-up to speculate whether serum endocan levels are associated with recurrence or progression of residue or upgrading of LGGs.
Limitations
The authors contributed to the present study acknowledge some limitations. The first and the most important limitation is that we included less number of patients. This is because of our strict inclusion criteria, and the majority of patients operated during the study period did not give permission to the study. Second limitation is that long-term serum samples were not obtained; it would be interesting to see if there any association between long-term serum levels of endocan and recurrence of tumor.
CONCLUSION
In conclusion, considering our limited findings, we can state that endocan can be detected in the serum of LGG patients, which may be novel, but potentially not meaningful. Because there is no study reported so far to show serum levels of endocan in glial tumor, it is difficult to speculate the role of endocan in LGGs. Future studies should aim to assess serum endocan levels before and after surgery in HGGs, which recur in very short period of time so that we will be able to have an idea about the recurrence and response to the treatment. We in this study just wanted to show how serum endocan levels change before and after surgery on LGGs.
Financial support and sponsorship
Nil.
Conflict of interest
The authors declare no conflict of interest. We would like to undertake the responsibility that the serum samples here are reused for the extension of our previous work.
References
1. Abid MR, Yi X, Yano K, Shih SC, Aird WC. Vascular endocan is preferentially expressed in tumor endothelium. Microvasc Res. 2006. 72: 136-45
2. Aldape K, Burger PC, Perry A. Clinicopathologic aspects of 1p/19q loss and the diagnosis of oligodendroglioma. Arch Pathol Lab Med. 2007. 131: 242-51
3. Delehedde M, Devenyns L, Maurage CA, Vives R. Endocan in cancers: A lesson from a circulating dermatan sulfate preteoglycan. Int J Cell Biol 2013. 2013. p. 705027-
4. Jiang H, Fu XG, Chen YT. Serum level of endothelial cell-specific molecule-1 and prognosis of colorectal cancer. Genet Mol Res. 2015. 14: 5519-26
5. Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg. 2001. 95: 190-8
6. Laloglu E, Aksoy H, Aksoy Y, Ozkaya F, Akcay F. Determination of serum and urinary endocan concentrations in patients with bladder cancer. Ann Clin Biochem. 2016. 53: 647-53
7. Lassalle P, Molet S, Janin A, Heyden JV, Tavernier J, Fiers W. ESM-1 is a novel human endothelial cell-specific molecule expressed in lung and regulated by cytokines. J Biol Chem. 2005. 271: 20458-64
8. Lote K, Egeland T, Hager B, Stenwig B, Skullerud K, Berg-Johnsen J. Survival, prognostic factors, and therapeutic efficacy in low-grade glioma: A retrospective study in 379 patients. J Clin Oncol. 1997. 15: 3129-40
9. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007. 114: 97-109
10. Lv Z, LV , Yongsheng F, Chen H, Zhao D. Endothelial cell-specific molecule-1: A potential serum marker for gastric cancer. Tumor Biol. 2014. 35: 10497-502
11. Matano F, Yoshida D, Ishii Y, Tahara S, Teramoto A, Morita A. Endocan, a new invasion and angiogenesis marker of pituitary adenoma. J Neurooncol. 2014. 173: 485-91
12. Maurage CA, Adam E, Mineo JF, Sarrazin S, Debunne M, Siminski RM. Endocan expression and localization in human glioblastoma. J Neuropathol Exp Neurol. 1999. 68: 633-41
13. Miao Y, Zong M, Jiang T, Yuan X, Guan S, Wang Y. A comparative analysis of ESM-1 and vascular endothelial cell marker (CD34/CD105) expression on pituitary adenoma invasion. Pituitary. 2016. 19: 194-201
14. Ozaki K, Toshikuni N, George J, Minato T, Matsue Y, Arisawa T. Serum endocan as a novel prognostic biomarker in patients with hepatocellular carcinoma. J Cancer. 2014. 5: 221-30
15. Scherpereel A, Depontieu F, Grigoriu B, Cavestri B, Tsicopoulos A, Gentina T. Endocan, a new endothelial marker in human sepsis. Crit Care Med. 2006. 34: 532-7
16. Zhu Y, Zhang X, Qi L, Cai Y, Yang P, Xuan G. HULC long noncoding RNA silencing suppresses angiogenesis by regulating ESM-1 via the PI3K/Akt/mTOR signaling pathway in human gliomas. Oncotarget. 2016. 7: 14429-40