- Departments of Neurosurgery and Interventional Neuroradiology, Cajuru University Hospital of Pontifical Catholic University (HUC-PUC), Curitiba, Parana, Brazil
Correspondence Address:
Luana Antunes Maranha Gatto
Departments of Neurosurgery and Interventional Neuroradiology, Cajuru University Hospital of Pontifical Catholic University (HUC-PUC), Curitiba, Parana, Brazil
DOI:10.4103/sni.sni_456_17
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Luana Antunes Maranha Gatto, Guilherme Naves de Lima Alves, Diego do Monte Rodrigues Seabra, Gelson Luis Koppe, Zeferino Demartini. Single-session percutaneous embolization with onyx and coils of sinus pericranii. 04-Jun-2018;9:114
How to cite this URL: Luana Antunes Maranha Gatto, Guilherme Naves de Lima Alves, Diego do Monte Rodrigues Seabra, Gelson Luis Koppe, Zeferino Demartini. Single-session percutaneous embolization with onyx and coils of sinus pericranii. 04-Jun-2018;9:114. Available from: http://surgicalneurologyint.com/surgicalint-articles/single%e2%80%91session-percutaneous-embolization-with-onyx-and-coils-of-sinus-pericranii/
Abstract
Background:Sinus pericranii (SP) is a rare vascular malformation consisting of an abnormal communication between the extra- and the intracranial venous system. It occurs due to the adhesion of vessels without a muscular layer or a hemangioma on the outer surface of the skull through diploic vessels, communicating with an intracranial venous sinus.
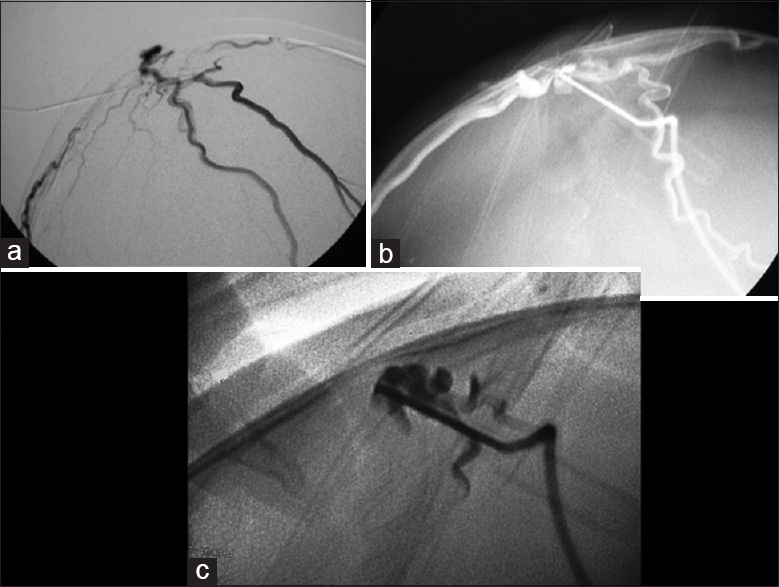
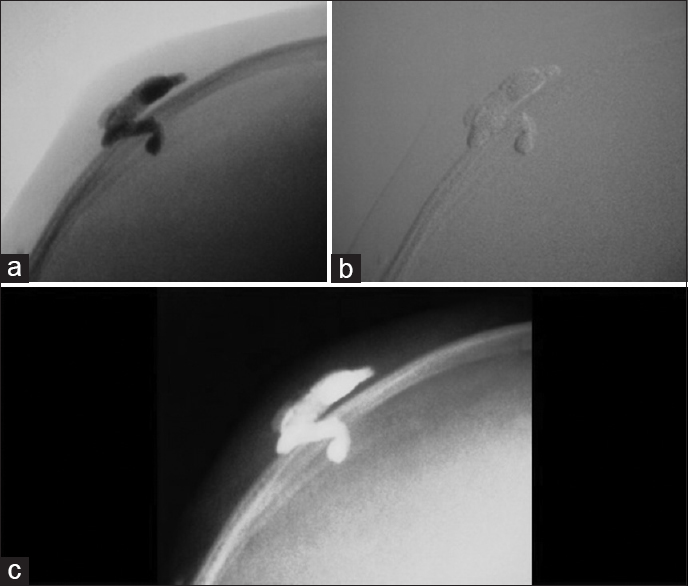
Case Description:A 10-month-old female presented with a pulsatile mass on the posterior parietal region. Investigation with brain vascular examinations showed a venous malformation communicating with the superior sagittal sinus under the scalp, without arterial feeder or nidus. An endovascular embolization with coils and a percutaneous embolization with Onyx ® were performed. The final venography showed complete exclusion of the lesion, which was gradually being absorbed.
Conclusion:This is the first case of an SP successfully treated in a single session by embolization with coils and onyx.
Keywords: Central nervous system vascular malformations, dural arteriovenous fistulas, endovascular procedures, sinus pericranii
INTRODUCTION
Sinus pericranii (SP) was first reported by Pott in 1760 when he discovered a soft tissue injury following a cranial fracture. As early as 1845, Hecker presented the disease with the name “varix spurius circumscriptus venae diploicae frontalis,” which became known as sinus pericranii only in 1850 when Stromeyer defined the disease as “a subperiosteal cystic sac filled with blood that communicates with an intracranial sinus.”[
SP is considered a rare benign venous abnormality, comprising an intradiploic emissary vein derived from an intracranial sinus,[
Due to the rarity of the disease, its pathogenesis and natural history remain obscure.[
Regarding gender distribution, some authors believe that there is a slight predominance in females,[
The diagnosis can be made with Doppler ultrasonography/computed tomography (CT)/magnetic resonance imaging (MRI)/magnetic resonance angiography (MRA)/angiotomography (CTA), and digital subtraction angiography (DSA), with DSA being the gold standard.[
An SP is considered true when changes in shape, size, or vascularity are observed with maneuvers that cause elevated intracranial pressure (ICP), such as Trendelenburg position, sudden neck flexion, jugular vein compression, Valsalva, cough, and crying.[
There is no scientific consensus about the choice of the best modality for treatment, which can include from open surgeries, such as the repair of the cranioestenosis and the manual ligation, until endovascular procedures, such as the percutaneous and transvenous embolization, or even percutaneous puncture direct and combined approaches.[
We report the case of a child with a large SP who was successfully treated only with embolization by percutaneous phlebography.
CASE REPORT
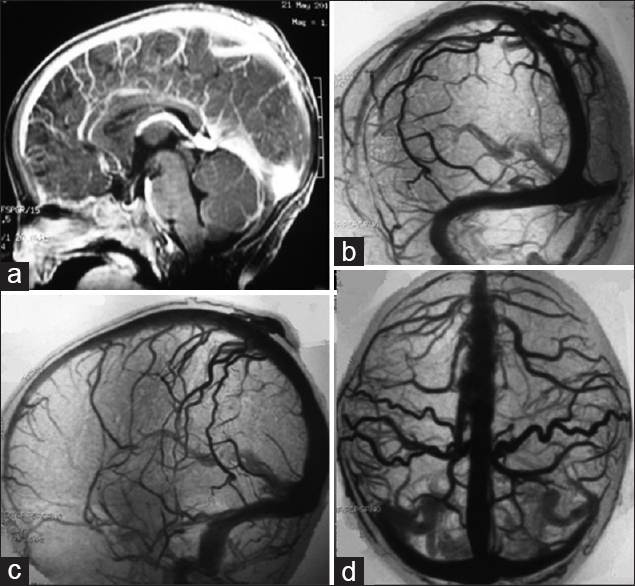
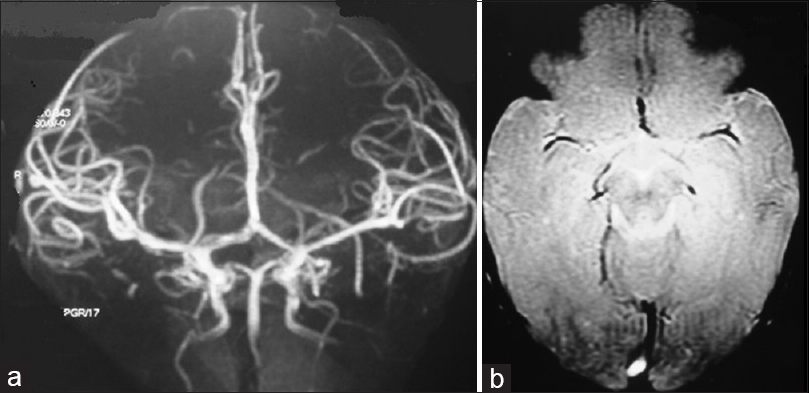
A 10-month-old female child presented with a pulsatile mass on the posterior parietal region with fremitus in the midline. There was no head trauma history and no neurological deficit on physical examination. Brain CT, MRI, MRA [
DISCUSSION
This is a report of an extremely rare disease. At present, there are just over 100 reported cases of SP in the literature.[
The lesion in our patient is a typical example of this disease – it was unique, was located in the midline in the parietal region, and measured 3 cm. The main location of the SP is midline or parasagittal (95%), distributed mainly in the frontal (43%) and parietal regions (36%). Other less common locations include occipital (8%), frontoparietal (5%), parietooccipital (3%), occipitotemporal (3%), and temporal (2%). The size of the conglomerate of extracranial vessels may range from 1 to 13 cm, more commonly between 2 and 6 cm. The skull defect varies between 1 and 4 mm, and large defects are rare.[
Like most cases, our patient was asymptomatic, with only complaints regarding the aesthetic defect. However, there are reports of diverse clinical presentation, from mild symptoms such as local pain, nausea, headache, feeling of pressure, and dizziness to more complex and serious symptoms such as abrupt increase in ICP, bradycardia, ataxia, and even bradycardia.[
Surgical excision, in a majority of cases, besides endovascular embolization, has positive results (cure, stabilization or emergencies/complications resolution).[
SPs are classified according to their vascular architecture, which helps in determining treatment approach. When interfering with SP circulation, the circulation of the associated dural venous sinus is also compromised, they are called dominant SP (25% of the cases). However, when the associated sinus vascularization does not change, regardless of the maneuver, SP is said accessory (75%).[
DSA plays a crucial role in the classification of SP and choice of optimal treatment. Although surgery is curative, some studies have reported significant hemorrhage because of dural sinus lacerations.[
Endovascular treatment of SP with N-butyl cyanoacrylate glue is possible, but we opted for the liquid embolic agent Onyx ® (ethylene-vinyl alcohol copolymer). This has been used in the endovascular treatment of intracranial arteriovenous malformations, arteriovenous fistulas, and other venous malformations with excellent results.[
There is risk to skin ulceration beyond pulmonary or systemic embolization. The latter could be minimized with use of “armored concrete” embolization (use of coils and Onyx).[
CONCLUSION
This is the first described case of SP treated in a single session by percutaneous embolization with coils (via endovascular) and Onyx (percutaneous). Although asymptomatic and only with an aesthetic objective, the treatment was well indicated when assessing the repercussion in the global venous drainage through angiography with maneuvers of compression of the scalp.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initial will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aburto-Murrieta DY, Bonifácio-Delgadillo JB, Bañares MJB, Castellanos AZ. Sinus Pericranii: Case Report. Vasc Endovasc Surg. 2010. 45: 103-5
2. Akram H, Preserakos G, Haliasos N, O'Donovan D, Low H. Sinus pericranii: an overview and literature review of a rare cranial venous anomaly (a review of the existing literature with case examples). Neurosurg Ver. 2012. 35: 15-26
3. Ellis JA, Mejia Munne JC, Feldstein NA, Meyers PM. Determination of sinus pericranii resectability by external compression during angiography: Technical note. J Neurosurg Pediatr. 2015. 16: 1-5
4. Khachatrian VA, Khodorovskaia AM, Sebelev KI, Zabrodskaia IuM. Pericranial sinus.Definition, diagnosis, surgical treatment. Zh Vopr Neirokhir Im N N Burdenko. 2014. 78: 30-7
5. Ni W, Tian Y, Gu Y, Mao Y. Transvenous Endovascular Treatment for Scalp Arteriovenous Fistulas: Results with Combined Use of Onyx and Coils. World Neurosurg. 2017. 107: 692-7
6. Osborn AG, Salzman LK, Jhaveri MD, Barkovich AJ.editors. Sinus Pericranii (section 6) In Diagnostic Imaging: Brain. Elsevier; 2016. p. 418-9
7. Park SC, Kim SK, Cho BK, Kim HJ, Kim JE, Phi JH. Sinus pericranii in children: Report of 16 patients and preoperative evaluation of surgical risk. J Neurosurg Pediatr. 2009. 4: 536-42
8. Pavanello M, Melloni I, Antichi E, Severino M, Ravegnani M, Piatelli G. Sinus pericranii: Diagnosis and management in 21 pediatric patients. Neurosurg Pediatr. 2015. 15: 60-70
9. Rangel-Castilla L, Krishna C, Klucznik R, Diaz O. Endovascular embolization with Onyx in the management of sinus pericranii: A case report. Neurosurg Focus. 2009. 27: E13-
10. Vinas FC, Valenzuela S, Zuleta A. Literature review: Sinus pericranii. Neurol Res. 1994. 16: 471-4