- Department of Neurosurgery, Faculty of Medicine, The University of Tokyo, Tokyo, Japan
- Department of Radiology, Faculty of Medicine, The University of Tokyo, Tokyo, Japan
Correspondence Address:
Satoru Miyawaki
Department of Neurosurgery, Faculty of Medicine, The University of Tokyo, Tokyo, Japan
DOI:10.4103/sni.sni_59_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hiroki Hongo, Satoru Miyawaki, Hideaki Imai, Yuki Shinya, Hideaki Ono, Harushi Mori, Hirofumi Nakatomi, Akira Kunimatsu, Nobuhito Saito. Smaller outer diameter of atherosclerotic middle cerebral artery associated with RNF213 c.14576G>A Variant (rs112735431). 05-Jun-2017;8:104
How to cite this URL: Hiroki Hongo, Satoru Miyawaki, Hideaki Imai, Yuki Shinya, Hideaki Ono, Harushi Mori, Hirofumi Nakatomi, Akira Kunimatsu, Nobuhito Saito. Smaller outer diameter of atherosclerotic middle cerebral artery associated with RNF213 c.14576G>A Variant (rs112735431). 05-Jun-2017;8:104. Available from: http://surgicalneurologyint.com/surgicalint-articles/smaller-outer-diameter-of-atherosclerotic-middle-cerebral-artery-associated-with-rnf213-c-14576ga-variant-rs112735431/
Abstract
Background:Intracranial atherosclerosis (ICAS) involves diverse histologies and several remodeling patterns. Ring finger protein 213 (RNF213) c.14576G>A variant (rs112735431), recently reported to be associated with ICAS, may be linked with negative remodeling (outer diameter – reducing morphological alteration) of intracranial arteries. This study investigated the outer diameter of atherosclerotic middle cerebral artery (MCA).
Methods:Patients with unilateral atherosclerotic MCA stenosis/occlusion were enrolled in this single-hospital-based case-control study at The University of Tokyo Hospital. The patients were divided into two groups by the presence of RNF213 c.14576G>A (variant group and wild-type group) and the outer diameter of the MCA was measured with high-resolution magnetic resonance imaging.
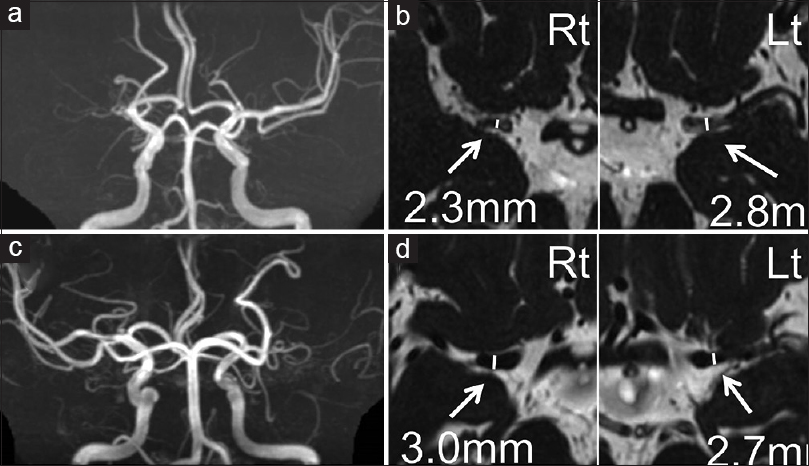
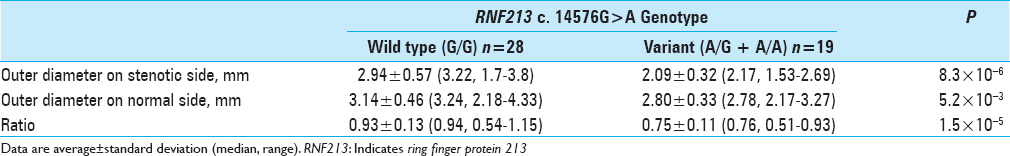
Results:Twenty-eight patients with the wild type and 19 patients with the variant type were included. The outer diameter of the stenotic side MCA was smaller in the variant group than in the wild-type group (P = 8.3 × 10-6). The outer diameter of the normal side MCA was also smaller in the variant group than in the wild-type group (P = 5.2 × 10-3). The ratio of stenotic side to normal side was also smaller in the variant group than in the wild-type group (P = 1.5 × 10-5).
Conclusions:This study indicates that RNF213 c.14576G>A is associated with negative remodeling of ICAS.
Keywords: Atherosclerosis, remodeling, genetics, intracranial artery stenosis, magnetic resonance imaging (MRI), RNF213
INTRODUCTION
Intracranial atherosclerosis (ICAS) is one of the main causes of ischemic stroke.[
Classification of arterial remodeling patterns is one of the widely used evaluation methods for arterial wall morphological characteristics. Progress in imaging technology such as high-resolution magnetic resonance imaging (MRI) has enabled the visualization of arterial morphological changes in ICAS,[
Recently, we identified a genetic variant that has a strong association with ICAS.[
RNF213 c.14576G>A was originally identified as a susceptibility gene variant of moyamoya disease (MMD), which is characterized by the progressive stenosis of the terminal portions of the bilateral internal carotid arteries.[
In the present study, we hypothesized that RNF213 c.14576G>A, the genetic variant associated with ICAS, is also associated with negative remodeling of ICAS. To prove this hypothesis, we analyzed the outer diameter of the intracranial arteries of ICAS patients divided into two groups according to the presence of RNF213 c.14576G>A.
MATERIALS AND METHODS
Patient population
This study prospectively enrolled patients with unilateral atherosclerotic middle cerebral artery (MCA) stenosis/occlusion who visited The University of Tokyo Hospital, Tokyo, Japan between April 2013 and December 2015. The criteria for inclusion were: (1) unilateral MCA (M1 portion) >50% stenosis/occlusion on magnetic resonance angiography (MRA); and (2) one or more risk factors of atherosclerosis including hypertension, diabetes mellitus, dyslipidemia, and history of cigarette smoking. Patients with non-atherosclerotic vasculopathy, such as dissection, vasculitis, or MMD, and evidence of cardioembolism were excluded. We also evaluated for the presence of symptoms. Symptomatic patients were defined as having both of MRI finding of cerebral ischemia in the distribution of the stenotic MCA and consistent focal neurological deficit.
MRI studies
MRI/MRA was performed in all patients. MRA was used to evaluate stenosis. Degree of luminal stenosis was classified into 5 intracranial artery stenosis (IAS) grades, according to a previously reported study, as: normal, no evidence of stenosis (grade 0); mild stenosis, <50% stenosis (grade 1); moderate stenosis, >50% stenosis (grade 2); severe stenosis, partial signal loss with the distal flow signal (grade 3); and occlusion, no distal flow signal (grade 4).[
Identification of RNF213 c.14576G>A variant (rs112735431)
Peripheral blood samples were obtained from all enrolled patients. Genomic DNA was obtained from the peripheral blood leukocytes at SRL, Inc. (Tachikawa, Tokyo, Japan) using a DNA extraction kit (Talent Srl, Trieste, Italy). Screening for the RNF213 c.14576G>A was performed by direct Sanger sequencing in all cases. RNF213 exon 61, which includes the c.14576G>A variant of RNF213 (GenBank accession number, NM_020914.4), was amplified by polymerase chain reaction (PCR). The primers 5′-CTGCATCACAGGAAATGACACTG and 5′-TGACGAGAAGAGCTTTCAGACGA were used for amplification and sequencing, as reported previously.[
Statistical analysis
The Pearson Chi-square test was used to compare the clinical characteristics between the wild type group (patients with RNF213 c.14576G>A wild type, GG) and variant group (patients with RNF213 c.14576G>A variant both heterozygote and homozygote, AG and AA). The Mann–Whitney U test was used to compare non-normally distributed continuous variables, such as age and diameter of the intracranial arteries between the two groups. All analyses were performed using JMP Pro version 11.0.0 (SAS Institute, Inc., Cary, NC). P value less than 0.05 was considered to be statistically significant.
Ethical considerations
This study was approved by the Human Genome, Gene Analysis Research Ethics Committee of the Faculty of Medicine, The University of Tokyo (approval number: 3516; approval date: September 12, 2011). Written informed consents were obtained from all participants in this study.
RESULTS
Clinical characteristics
Outer diameter
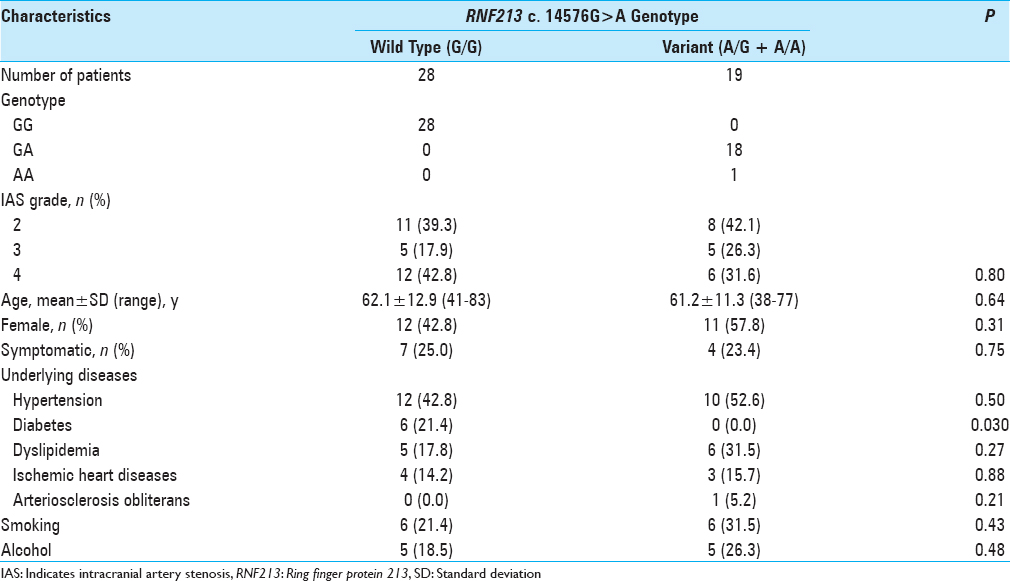
Figure 1
Representative cases are presented. MRA (a and c) and FIESTA images (b and d) of a 70-year-old man with RNF213 variant (a and b) and of a 72-year-old woman without RNF213 variant (c and d). Outer diameters of the bilateral M1 are shown. >50% stenosis or occlusion cases on MRA were included and the outer diameter of M1 was measured at the greatest minor axis in the proximal portion on axial FIESTA MRI. Rt indicates right side; Lt, left side
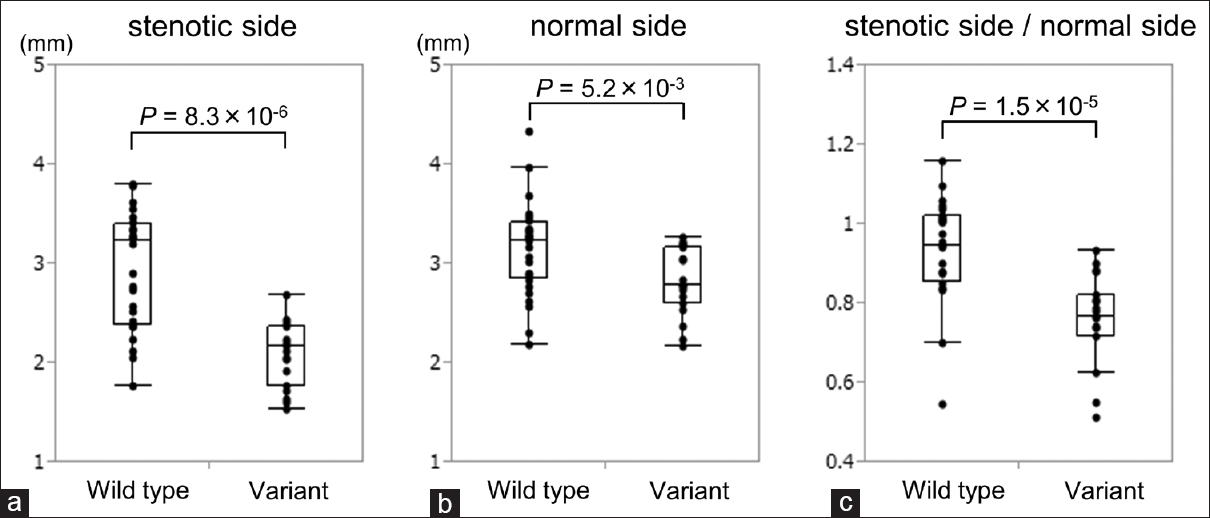
Figure 2
(a) A box plot of the outer diameters of M1 on the stenotic side of wild type and variant groups. Variant group had significantly smaller outer diameter on the involved side than the wild type group. (b) A box plot of the outer diameters of M1 on the normal side. The variant group also had significantly smaller outer diameter on the normal side than wild type group. (c) A box plot of the ratio of outer diameters of M1 on the stenotic side to the normal side. Variant group had significantly smaller ratio than the wild type group
DISCUSSION
The present study found that the outer diameter of MCA atherosclerosis was smaller in patients with the RNF213 c.14576G>A than in patients without the RNF213 c.14576G>A. In addition, the ratio of the outer diameter of the stenotic side to the normal side in MCA atherosclerosis was smaller in patients with the RNF213 c.14576G>A than in patients without RNF213 c.14576G>A. These results indicate that RNF213 c.14576G>A is associated with negative arterial remodeling in ICAS.
The histology of positive remodeling of atherosclerotic arteries in ICAS as well as other systemic arteries is known to involve lipid-rich plaque burden, intraplaque hemorrhage, fibrin cap, and infiltration of inflammatory cells.[
The smaller outer diameter on the normal side in the variant group indicates that the RNF213 c.14576G>A affects the outer diameter of normal intracranial arteries. Consequently, the present study identified RNF213 c.14576G>A as a genetic factor in the structure of normal intracranial artery. This important result suggests that the RNF213 c.14576G>A may affect either the morphogenesis or the morphological change of the intracranial artery. The present study investigated only ICAS patients classified by the presence of RNF213 c.14576G>A, hence further study should compare the character-matched groups of healthy individuals classified by the presence of RNF213 c.14576G>A to confirm the influence of RNF213 c.14576G>A on the normal intracranial artery.
RNF213 encodes a protein with 5256 amino acids harboring a RING (really interesting new gene) finger motif and an AAA (adenosine triphosphatase associated with various cellular activities) domain, indicating the presence of both E3 ubiquitin ligase activity and energy-dependent unfoldase.[
Remodeling patterns are associated with the risk of ischemic stroke,[
The present study has some limitations. Only the outer diameter was measured on MRI as the morphological feature of ICAS and wall structure itself was not investigated. Wall imaging could observe more detailed influence of the RNF213 c.14576G>A on the morphological characteristics of ICAS. In addition, to evaluate the effect of RNF213 c.14576G>A on the normal intracranial artery, character-matched groups classified by the presence of RNF213 c.14576G>A of healthy individuals, not only of atherosclerotic patients, must be compared.
CONCLUSION
This study indicates that RNF213 c.14576G>A is associated with negative remodeling of ICAS. Identification of the RNF213 c.14576G>A may lead to optimum treatment of ICAS.
Financial support and sponsorship
This work was supported by a Grant-in-Aid for Scientific Research (B) (No. 25293304) to Dr. Saito; and a Grant-in-Aid for Young Scientists (B) (No. 15K19949) from the Japan Society for the Promotion of Science and grants from Mitsui Life Social Welfare Foundation, Tokyo, Japan to Dr. Miyawaki.
Conflicts of interest
There are no conflicts of interest.
References
1. Ahn SH, Lee J, Kim YJ, Kwon SU, Lee D, Jung SC. Isolated MCA disease in patients without significant atherosclerotic risk factors: A high-resolution magnetic resonance imaging study. Stroke. 2015. 46: 697-703
2. Arenillas JF. Intracranial atherosclerosis: Current concepts. Stroke. 2011. 42: S20-3
3. Bruno R, Barzacchi M, Cartoni G, Antonelli M, Taddei S, Ghiadoni L. 4d.06: Progression of carotid artery remodeling and stiffness in hypertensive patients: A prospective cohort study. J Hypertens. 2015. 33: e62-
4. Burke AP, Kolodgie FD, Farb A, Weber D, Virmani R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation. 2002. 105: 297-303
5. Chen XY, Wong KS, Lam WW, Zhao HL, Ng HK. Middle cerebral artery atherosclerosis: Histological comparison between plaques associated with and not associated with infarct in a postmortem study. Cerebrovasc Dis. 2008. 25: 74-80
6. Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011. 365: 993-1003
7. Choi YJ, Jung SC, Lee DH. Vessel Wall Imaging of the Intracranial and Cervical Carotid Arteries. J Stroke. 2015. 17: 238-55
8. Chung GH, Kwak HS, Hwang SB, Jin GY. High resolution MR imaging in patients with symptomatic middle cerebral artery stenosis. Eur J Radiol. 2012. 81: 4069-74
9. Harteveld AA, Denswil NP, Siero JC, Zwanenburg JJ, Vink A, Pouran B. Quantitative Intracranial Atherosclerotic Plaque Characterization at 7T MRI: An Ex Vivo Study with Histologic Validation. AJNR Am J Neuroradiol. 2016. 37: 802-10
10. Hitomi T, Habu T, Kobayashi H, Okuda H, Harada KH, Osafune K. Downregulation of Securin by the variant RNF213 R4810K (rs112735431, G>A) reduces angiogenic activity of induced pluripotent stem cell-derived vascular endothelial cells from moyamoya patients. Biochem Biophys Res Commun. 2013. 438: 13-9
11. Jiang WJ, Cheng-Ching E, Abou-Chebl A, Zaidat OO, Jovin TG, Kalia J. Multicenter analysis of stenting in symptomatic intracranial atherosclerosis. Neurosurgery. 2012. 70: 25-30
12. Jiang Y, Zhu C, Peng W, Degnan AJ, Chen L, Wang X. Ex-vivo imaging and plaque type classification of intracranial atherosclerotic plaque using high resolution MRI. Atherosclerosis. 2016. 249: 10-6
13. Kaku Y, Morioka M, Ohmori Y, Kawano T, Kai Y, Fukuoka H. Outer-diameter narrowing of the internal carotid and middle cerebral arteries in moyamoya disease detected on 3D constructive interference in steady-state MR image: Is arterial constrictive remodeling a major pathogenesis?. Acta Neurochir. 2012. 154: 2151-7
14. Kamada F, Aoki Y, Narisawa A, Abe Y, Komatsuzaki S, Kikuchi A. A genome-wide association study identifies RNF213 as the first Moyamoya disease gene.J Hum Genet. 2011. 56: 34-40
15. Kanoke A, Fujimura M, Niizuma K, Ito A, Sakata H, Sato-Maeda M. Temporal profile of the vascular anatomy evaluated by 9.4-tesla magnetic resonance angiography and histological analysis in mice with the R4859K mutation of RNF213, the susceptibility gene for moyamoya disease. Brain Res. 2015. 1624: 497-505
16. Kobayashi H, Matsuda Y, Hitomi T, Okuda H, Shioi H, Matsuda T. Biochemical and Functional Characterization of RNF213 (Mysterin) R4810K, a Susceptibility Mutation of Moyamoya Disease, in Angiogenesis In Vitro and In Vivo. J Am Heart Assoc. 2015. 4: e002146-
17. Koo J. The Latest Information on Intracranial Atherosclerosis: Diagnosis and Treatment. Interv Neurol. 2015. 4: 48-50
18. Kume T, Okura H, Kawamoto T, Akasaka T, Toyota E, Watanabe N. Relationship between coronary remodeling and plaque characterization in patients without clinical evidence of coronary artery disease. Atherosclerosis. 2008. 197: 799-805
19. Kuroda S, Kashiwazaki D, Akioka N, Koh M, Hori E, Nishikata M. Specific Shrinkage of Carotid Forks in Moyamoya Disease: A Novel Key Finding for Diagnosis. Neurol Med Chir (Tokyo). 2015. 55: 796-804
20. Kurosaki Y, Yoshida K, Fukumitsu R, Sadamasa N, Handa A, Chin M. Carotid artery plaque assessment using quantitative expansive remodeling evaluation and MRI plaque signal intensity. J Neurosurg. 2016. 124: 736-42
21. Liu W, Morito D, Takashima S, Mineharu Y, Kobayashi H, Hitomi T. Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development. PLoS One. 2011. 6: e22542-
22. Miyatake S, Miyake N, Touho H, Nishimura-Tadaki A, Kondo Y, Okada I. Homozygous c.14576G>A variant of RNF213 predicts early-onset and severe form of moyamoya disease. Neurology. 2012. 78: 803-10
23. Miyawaki S, Imai H, Shimizu M, Yagi S, Ono H, Mukasa A. Genetic variant RNF213 c.14576G>A in various phenotypes of intracranial major artery stenosis/occlusion. Stroke. 2013. 44: 2894-7
24. Miyawaki S, Imai H, Takayanagi S, Mukasa A, Nakatomi H, Saito N. Identification of a genetic variant common to moyamoya disease and intracranial major artery stenosis/occlusion. Stroke. 2012. 43: 3371-4
25. Pasterkamp G, Hillen B, Borst C. Arterial remodelling by atherosclerosis. Semin Interv Cardiol. 1997. 2: 147-52
26. Pasterkamp G, Wensing PJ, Post MJ, Hillen B, Mali WP, Borst C. Paradoxical arterial wall shrinkage may contribute to luminal narrowing of human atherosclerotic femoral arteries. Circulation. 1995. 91: 1444-9
27. Prati F, Mallus MT, Parma A, Lioy E, Pagano A, Boccanelli A. Incidence of compensatory enlargement and paradoxical shrinkage of coronary arteries in presence of atherosclerotic lesions: An intracoronary ultrasound study based on multiple cross-section analysis per artery. G Ital Cardiol. 1998. 28: 1063-71
28. Qiao Y, Anwar Z, Intrapiromkul J, Liu L, Zeiler SR, Leigh R. Patterns and Implications of Intracranial Arterial Remodeling in Stroke Patients. Stroke. 2016. 47: 434-40
29. Ryoo S, Cha J, Kim SJ, Choi JW, Ki CS, Kim KH. High-resolution magnetic resonance wall imaging findings of Moyamoya disease. Stroke. 2014. 45: 2457-60
30. Ryoo S, Lee MJ, Cha J, Jeon P, Bang OY. Differential Vascular Pathophysiologic Types of Intracranial Atherosclerotic Stroke: A High-Resolution Wall Magnetic Resonance Imaging Study. Stroke. 2015. 46: 2815-21
31. Saam T, Habs M, Buchholz M, Schindler A, Bayer-Karpinska A, Cyran CC. Expansive arterial remodeling of the carotid arteries and its effect on atherosclerotic plaque composition and vulnerability: An in-vivo black-blood 3T CMR study in symptomatic stroke patients. J Cardiovasc Magn Reson. 2016. 18: 11-
32. Schoenhagen P, Ziada KM, Vince DG, Nissen SE, Tuzcu EM. Arterial remodeling and coronary artery disease: The concept of “dilated” versus “obstructive” coronary atherosclerosis. J Am Col Cardiol. 2001. 38: 297-306
33. Scholz B, Korn C, Wojtarowicz J, Mogler C, Augustin I, Boutros M. Endothelial RSPO3 Controls Vascular Stability and Pruning through Non-canonical WNT/Ca (2+)/NFAT Signaling. Dev Cell. 2016. 36: 79-93
34. Smits PC, Bos L, Quarles van Ufford MA, Eefting FD, Pasterkamp G, Borst C. Shrinkage of human coronary arteries is an important determinant of de novo atherosclerotic luminal stenosis: An in vivo intravascular ultrasound study. Heart. 1998. 79: 143-7
35. Sonobe S, Fujimura M, Niizuma K, Nishijima Y, Ito A, Shimizu H. Temporal profile of the vascular anatomy evaluated by 9.4-T magnetic resonance angiography and histopathological analysis in mice lacking RNF213: A susceptibility gene for moyamoya disease. Brain Res. 2014. 1552: 64-71
36. Suri MF, Qiao Y, Ma X, Guallar E, Zhou J, Zhang Y. Prevalence of Intracranial Atherosclerotic Stenosis Using High-Resolution Magnetic Resonance Angiography in the General Population: The Atherosclerosis Risk in Communities Study. Stroke. 2016. 47: 1187-93
37. Uchiyama S, Sakai N, Toi S, Ezura M, Okada Y, Takagi M. Final Results of Cilostazol-Aspirin Therapy against Recurrent Stroke with Intracranial Artery Stenosis (CATHARSIS). Cerebrovasc Dis Extra. 2015. 5: 1-13
38. Varnava AM, Mills PG, Davies MJ. Relationship between coronary artery remodeling and plaque vulnerability. Circulation. 2002. 105: 939-43
39. Wexberg P, Gyongyosi M, Sperker W, Kiss K, Yang P, Hassan A. Pre-existing arterial remodeling is associated with in-hospital and late adverse cardiac events after coronary interventions in patients with stable angina pectoris. J Am Col Cardiol. 2000. 36: 1860-9
40. Xu P, Lv L, Li S, Ge H, Rong Y, Hu C. Use of high-resolution 3.0-T magnetic resonance imaging to characterize atherosclerotic plaques in patients with cerebral infarction. Exp Ther Med. 2015. 10: 2424-8
41. Xu WH, Li ML, Gao S, Ni J, Zhou LX, Yao M. In vivo high-resolution MR imaging of symptomatic and asymptomatic middle cerebral artery atherosclerotic stenosis. Atherosclerosis. 2010. 212: 507-11
42. Yamagishi M, Terashima M, Awano K, Kijima M, Nakatani S, Daikoku S. Morphology of vulnerable coronary plaque: Insights from follow-up of patients examined by intravascular ultrasound before an acute coronary syndrome. J Am Coll Cardiol. 2000. 35: 106-11
43. Yang WJ, Chen XY, Zhao HL, Niu CB, Xu Y, Wong KS. In vitro Assessment of Histology Verified Intracranial Atherosclerotic Disease by 1.5T Magnetic Resonance Imaging: Concentric or Eccentric?. Stroke. 2016. 47: 527-30
44. Zhao DL, Deng G, Xie B, Ju S, Yang M, Chen XH. High-resolution MRI of the vessel wall in patients with symptomatic atherosclerotic stenosis of the middle cerebral artery. J Clin Neurosci. 2015. 22: 700-4
45. Zhu XJ, Du B, Lou X, Hui FK, Ma L, Zheng BW. Morphologic characteristics of atherosclerotic middle cerebral arteries on 3T high-resolution MRI. AJNR Am J Neuroradiol. 2013. 34: 1717-22
46. Zhu X, Liu L, He X, Zhang X, Hu L, Du B. Wall thickening pattern in atherosclerotic basilar artery stenosis. Neurol Sci. 2016. 37: 269-76