- Department of Neurologic Surgery, Vanderbilt University Medical Center, Nashville, TN, USA
Correspondence Address:
Rebecca A. Kasl
Department of Neurologic Surgery, Vanderbilt University Medical Center, Nashville, TN, USA
DOI:10.4103/2152-7806.181985
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kasl RA, Brinson PR, Chambless LB. Socioeconomic status does not affect prognosis in patients with glioblastoma multiforme. Surg Neurol Int 06-May-2016;7:
How to cite this URL: Kasl RA, Brinson PR, Chambless LB. Socioeconomic status does not affect prognosis in patients with glioblastoma multiforme. Surg Neurol Int 06-May-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/socioeconomic-status-does-not-affect-prognosis-in-patients-with-glioblastoma-multiforme/
Abstract
Background:Glioblastoma multiforme (GBM) is an aggressive malignancy, but there is marked heterogeneity in survival time. Health care disparities have demonstrated significance in oncologic outcomes but have not been clearly examined in this patient population. We investigated the role of sociodemographic variables in the prognosis of adult patients diagnosed with GBM.
Methods:This retrospective analysis included patients with a histologically confirmed diagnosis of GBM, who underwent resection or biopsy at a single institution from 2000 to 2014. Socioeconomic status (SES) was determined by household income according to the US Census zip code tabulation areas and the US national poverty level. Multivariate Cox proportional hazards analysis calculated effects on patient survival.
Results:Thirty percent of 218 subjects were of low SES, 57% mid, and 13% high. Low SES patients tended to be male (62%), Caucasian (92%), unmarried (91%), have dependents (100%), and limited to high school education (55%). SES did not predict insurance or employment status. SES was associated with marital status and number of cohabitants (P P = 0.0125), elderly patients (HR 1.70, P = 0.0158), and multifocal disease (HR 1.75, P = 0.0119). Longer prognosis was associated with gross total resection (HR 0.49, P = 0.0009), radiation therapy (HR 0.12, P P
Conclusions:SES alone does not predict prognosis in patients with newly diagnosed GBM. Sociodemographic variables such as old age, military service record, and insurance type may have a prognostication role.
Keywords: Brain tumor, glioblastoma multiforme, glioma, poverty, prognosis, socioeconomic status
INTRODUCTION
Gliomas are the most common type of primary brain tumor.[
Socioeconomic status (SES) has been previously suggested to affect outcomes in a variety of malignancies.[
The zip code has been frequently used in US-based public health research as a proxy for SES.[
Using ZCTAs and government-sanctioned national poverty levels, we sought to assess the role of SES and other demographic variables in the prognosis of adult patients diagnosed with GBM.
METHODS
We retrospectively analyzed patients with a histologically confirmed diagnosis of GBM, who underwent resection or biopsy at Vanderbilt University Medical Center from 2000 to 2014. Children and incarcerated patients were excluded from the study. Two hundred eighteen subjects were included. After approval from the Vanderbilt Institutional Review Board, data were extracted from electronic medical records and cataloged in the Research Electronic Data Capture database.[
To improve internal validity, zip codes were transformed into ZCTA codes in accordance with the 2012 United States Census.[
All data were de-identified before statistical analysis in Microsoft Excel (Microsoft. Redmond, Washington)[
RESULTS
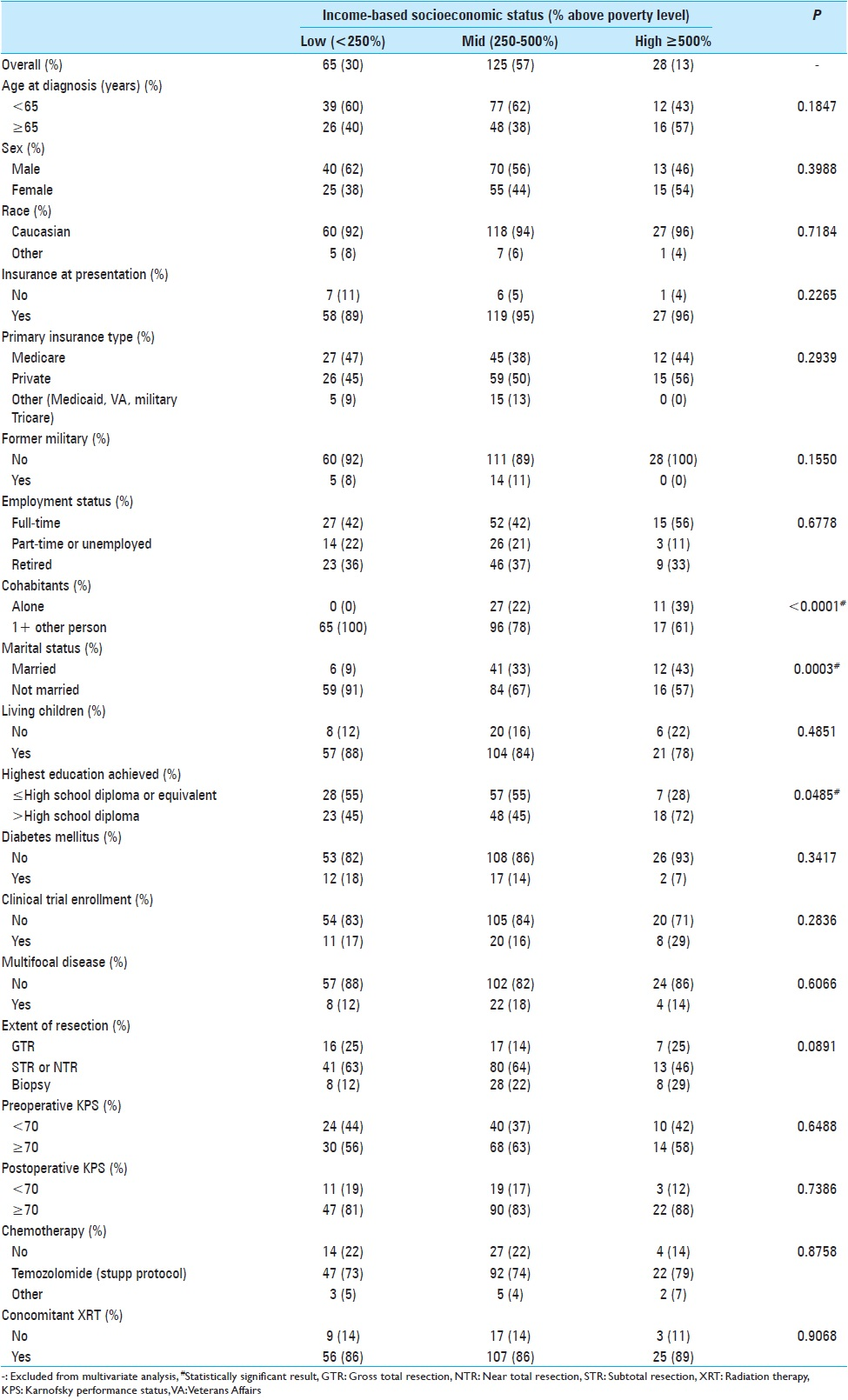
The 218 study subjects were stratified according to their income-based socioeconomic profile [
SES predicted marital status (P = 0.0003), number of cohabitants (P < 0.0001), and level of education attained (P = 0.0485). Our institution makes a concentrated effort to provide healthcare to the underserved, and most patients in this study were of low SES (47%). There was no statistical different in race, age, or sex across SES. Low SES patients were more likely to be unmarried, live with at least 1 other person in their home, and have less than or equal to a high school education. SES did not predict presence of insurance or employment status. Low SES patients were not more likely to have Medicaid insurance.
All patients had a histologically confirmed diagnosis of Grade IV astrocytoma. Multifocal disease presentation was uncommon (16%). Most patients (61%) received near total resection or subtotal resection. Extent of resection, KPS, clinical trial enrollment, and type of treatment received were not predicted by SES. Overall patient functional status pre- and post-operatively was high. The preoperative and postoperative KPS scores were at least 70 in 85% and 88% of patients, respectively. Seventy-five percent received TMZ according to the Stupp protocol, and 87% were treated with XRT.
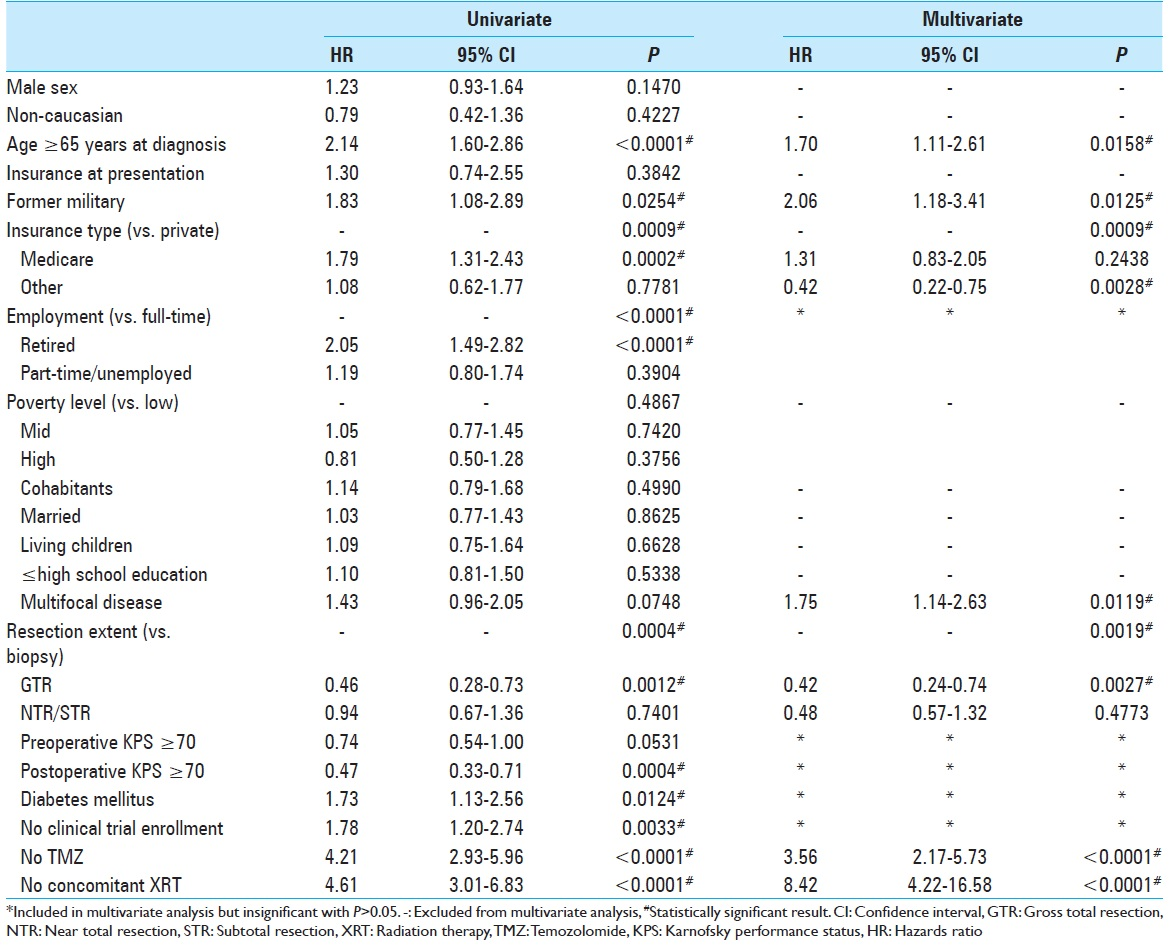
Univariate Cox proportional hazards analysis yielded 12 variables with P < 0.10 to meet inclusion criteria for the multivariate analysis [
DISCUSSION
With the rising costs of healthcare and increasing incidence of cancer, oncology patients make up a significant component of the national disease burden in the United States. A patient's ability to access and afford treatment is affected by their social situation and economic resources. In the oncology setting, research calls into question the impact of SES on overall survival after a diagnosis of cancer.[
Sociodemographic variables are relevant to both the developing and developed world. They have an established role in many different types of malignancies and various stages throughout the disease course.[
Race is a frequently studied demographic variable in oncology. African-American patients have decreased overall survival in non-central nervous system (CNS) adult and pediatric malignancies compared to their Caucasian counterparts.[
Socioeconomic data specific to CNS malignancy incidence and outcomes have yielded mixed results.[
ZCTA codes served as a useful representation of income and proxy for SES in our study. Other studies in and outside of neurosurgery have used zip codes as an SES marker as well.[
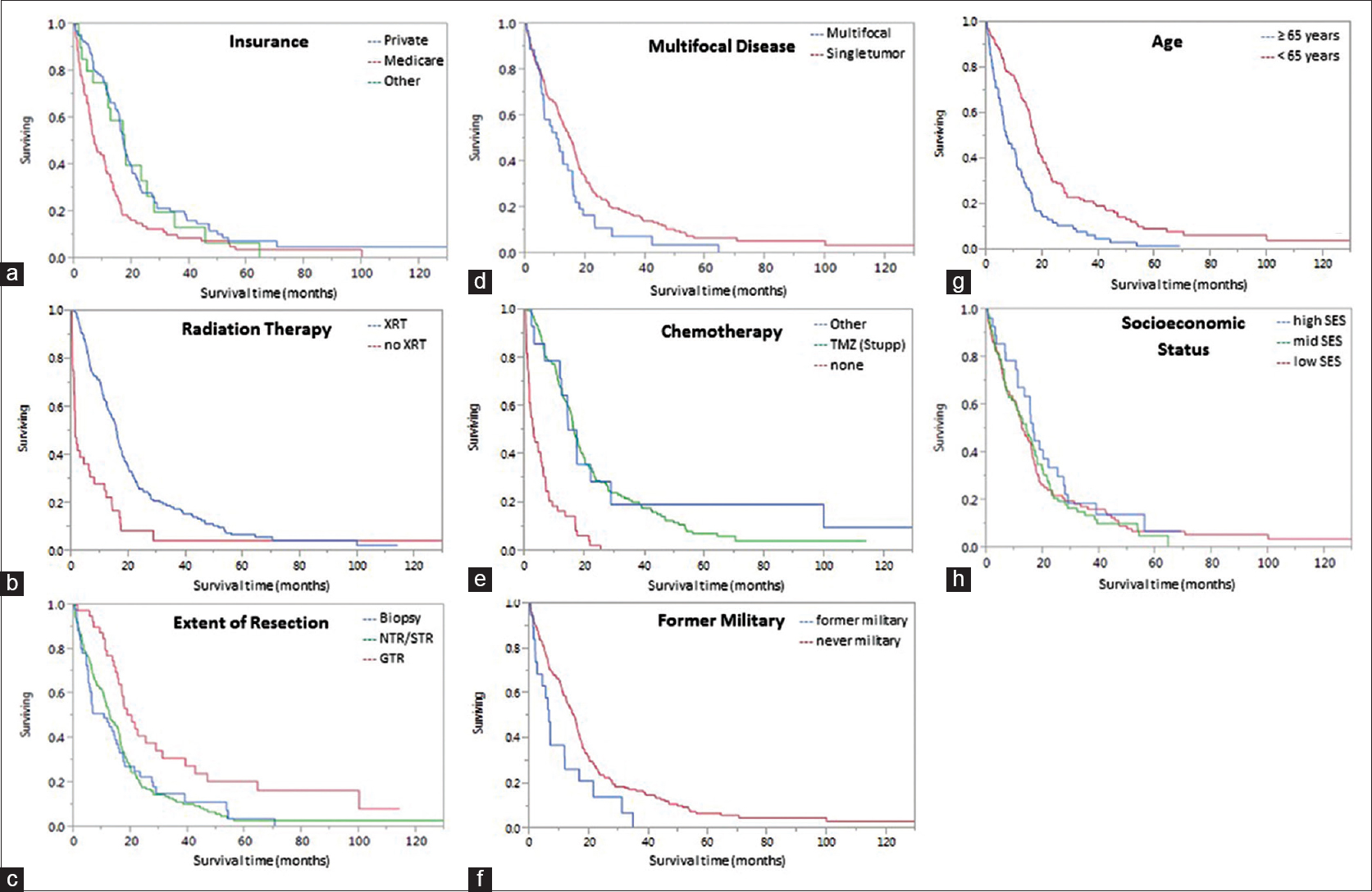
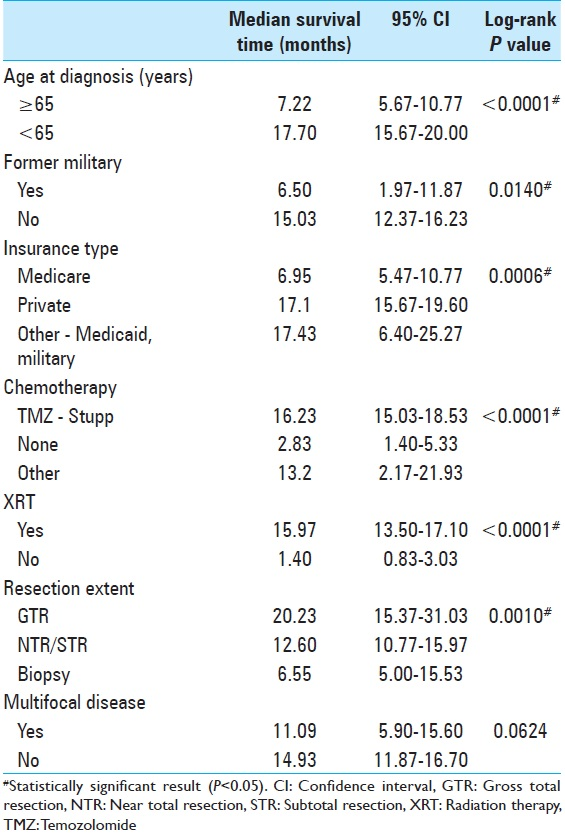
Patient resources were further analyzed in our study of military background and insurance status at the time of diagnosis. While lack of insurance did not affect prognosis, a patient's insurance type was statistically significant in the multivariate model [
Glioblastoma has been studied extensively in search of predictors separating the short from long-term survivors. No factor has been found to more strongly affect tumor prognosis than the addition of TMZ to XRT since the 2005 Stupp trial;[
We disproved our hypothesis and found SES to not be associated with GBM prognosis in a sample size whose demographics were similar to the local population. This is notably different from several other malignancies; in which, authors have presented a prognostic role for sociodemographic variables.[
CONCLUSIONS
In our US-based study using ZCTA codes as a proxy for SES, we present new data demonstrating that SES does not affect GBM prognosis. However, sociodemographic variables such as a military service record and insurance type have a suggested prognostication role. Consistent with prior studies, old age, multifocal tumors, XRT, and chemotherapy affected outcomes. XRT has the largest impact on median survival time. Although a small, single-institution, retrospective study, this research presents a formative opportunity for physicians to consider a patient's socioeconomic profile when treating GBM.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Travis Ladner and Dr. Edmond Kabagambe for their assistance with the statistical analysis.
References
1. Last accessed on 2015 May 05. Available from: http://www.aspe.hhs.gov/poverty/12povertys.html.
2. Ansbaugh N, Shannon J, Mori M, Farris PE, Garzotto M. Agent orange as a risk factor for high-grade prostate cancer. Cancer. 2013. 119: 2399-404
3. Austin MT, Nguyen H, Eberth JM, Chang Y, Heczey A, Hughes DP. Health disparities are important determinants of outcome for children with solid tumor malignancies. J Pediatr Surg. 2015. 50: 161-6
4. Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002. 287: 2106-13
5. Barbano R, Palumbo O, Pasculli B, Galasso M, Volinia S, D’Angelo V. A miRNA signature for defining aggressive phenotype and prognosis in gliomas. PLoS One. 2014. 9: e108950-
6. Barnholtz-Sloan JS, Sloan AE, Schwartz AG. Relative survival rates and patterns of diagnosis analyzed by time period for individuals with primary malignant brain tumor, 1973-1997. J Neurosurg. 2003. 99: 458-66
7. Barnholtz-Sloan JS, Williams VL, Maldonado JL, Shahani D, Stockwell HG, Chamberlain M. Patterns of care and outcomes among elderly individuals with primary malignant astrocytoma. J Neurosurg. 2008. 108: 642-8
8. Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M. Socioeconomic status in health research: One size does not fit all. JAMA. 2005. 294: 2879-88
9. Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol. 2003. 30: 10-4
10. Byers TE, Wolf HJ, Bauer KR, Bolick-Aldrich S, Chen VW, Finch JL. The impact of socioeconomic status on survival after cancer in the United States: Findings from the National Program of Cancer Registries Patterns of Care Study. Cancer. 2008. 113: 582-91
11. Chaichana KL, Pendleton C, Chambless L, Camara-Quintana J, Nathan JK, Hassam-Malani L. Multi-institutional validation of a preoperative scoring system which predicts survival for patients with glioblastoma. J Clin Neurosci. 2013. 20: 1422-6
12. Chakrabarti I, Cockburn M, Cozen W, Wang YP, Preston-Martin S. A population-based description of glioblastoma multiforme in Los Angeles County, 1974-1999. Cancer. 2005. 104: 2798-806
13. Chambless LB, Kistka HM, Parker SL, Hassam-Malani L, McGirt MJ, Thompson RC. The relative value of postoperative versus preoperative Karnofsky Performance Scale scores as a predictor of survival after surgical resection of glioblastoma multiforme. J Neurooncol. 2015. 121: 359-64
14. Chambless LB, Parker SL, Hassam-Malani L, McGirt MJ, Thompson RC. Type 2 diabetes mellitus and obesity are independent risk factors for poor outcome in patients with high-grade glioma. J Neurooncol. 2012. 106: 383-9
15. Cominelli M, Grisanti S, Mazzoleni S, Branca C, Buttolo L, Furlan D. EGFR amplified and overexpressing glioblastomas and association with better response to adjuvant metronomic temozolomide. J Natl Cancer Inst. 2015. 107:
16. Curry WT, Barker FG. Racial, ethnic and socioeconomic disparities in the treatment of brain tumors. J Neurooncol. 2009. 93: 25-39
17. Dalton SO, Steding-Jessen M, Jakobsen E, Mellemgaard A, Østerlind K, Schüz J. Socioeconomic position and survival after lung cancer: Influence of stage, treatment and comorbidity among Danish patients with lung cancer diagnosed in 2004-2010. Acta Oncol. 2015. 54: 797-804
18. Demers PA, Vaughan TL, Schommer RR. Occupation, socioeconomic status, and brain tumor mortality: A death certificate-based case-control study. J Occup Med. 1991. 33: 1001-6
19. Derakhshan A, Miller J, Lubelski D, Nowacki AS, Wells BJ, Milinovich A. The impact of socioeconomic status on the utilization of spinal imaging. Neurosurgery. 2015. 77: 746-53
20. . Disparities in cancer care. J Oncol Pract. 2006. 2: 234-90
21. Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol. 2012. 14: v1-49
22. Field KM, Drummond KJ, Yilmaz M, Tacey M, Compston D, Gibbs P. Clinical trial participation and outcome for patients with glioblastoma: Multivariate analysis from a comprehensive dataset. J Clin Neurosci. 2013. 20: 783-9
23. Field KM, Rosenthal MA, Yilmaz M, Tacey M, Drummond K. Comparison between poor and long-term survivors with glioblastoma: Review of an Australian dataset. Asia Pac J Clin Oncol. 2014. 10: 153-61
24. Gentil J, Dabakuyo TS, Ouedraogo S, Poillot ML, Dejardin O, Arveux P. For patients with breast cancer, geographic and social disparities are independent determinants of access to specialized surgeons. A eleven-year population-based multilevel analysis. BMC Cancer. 2012. 12: 351-
25. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009. 42: 377-81
26. Herndon JE, Kornblith AB, Holland JC, Paskett ED. Effect of socioeconomic status as measured by education level on survival in breast cancer clinical trials. Psychooncology. 2013. 22: 315-23
27. Keles GE, Anderson B, Berger MS. The effect of extent of resection on time to tumor progression and survival in patients with glioblastoma multiforme of the cerebral hemisphere. Surg Neurol. 1999. 52: 371-9
28. Krieger N, Waterman P, Chen JT, Soobader MJ, Subramanian SV, Carson R. Zip code caveat: Bias due to spatiotemporal mismatches between zip codes and US census-defined geographic areas – The public health disparities geocoding project. Am J Public Health. 2002. 92: 1100-2
29. Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: A population-based, linked database analysis of 396 patients. Ann Surg. 2003. 237: 74-85
30. Long B, Chang J, Ziogas A, Tewari KS, Anton-Culver H, Bristow RE. Impact of race, socioeconomic status, and the health care system on the treatment of advanced-stage ovarian cancer in California. Am J Obstet Gynecol. 2015. 132: 221-6
31. Lynch JC, Welling L, Escosteguy C, Pereira AG, Andrade R, Pereira C. Socioeconomic and educational factors interference in the prognosis for glioblastoma multiform. Br J Neurosurg. 2013. 27: 80-3
32. Mahal BA, Inverso G, Aizer AA, Ziehr DR, Hyatt AS, Choueiri TK. Incidence and determinants of 1-month mortality after cancer-directed surgery. Ann Oncol. 2015. 26: 399-406
33. Mazaris P, Hong X, Altshuler D, Schultz L, Poisson LM, Jain R. Key determinants of short-term and long-term glioblastoma survival: A 14-year retrospective study of patients from the Hermelin Brain Tumor Center at Henry Ford Hospital. Clin Neurol Neurosurg. 2014. 120: 103-12
34. McLendon RE, Robinson JS, Chambers DB, Grufferman S, Burger PC. The glioblastoma multiforme in Georgia, 1977-1981. Cancer. 1985. 56: 894-7
35. .editors. Microsoft Excel, 2011. Redmond, WA: Microsoft Corporation; 2011. p.
36. Mukherjee D, Patil CG, Todnem N, Ugiliweneza B, Nuño M, Kinsman M. Racial disparities in Medicaid patients after brain tumor surgery. J Clin Neurosci. 2013. 20: 57-61
37. Patil CG, Yi A, Elramsisy A, Hu J, Mukherjee D, Irvin DK. Prognosis of patients with multifocal glioblastoma: A case-control study. J Neurosurg. 2012. 117: 705-11
38. Petridou ET, Sergentanis TN, Perlepe C, Papathoma P, Tsilimidos G, Kontogeorgi E. Socioeconomic disparities in survival from childhood leukemia in the United States and globally: A meta-analysis. Ann Oncol. 2015. 26: 589-97
39. Porter AB, Lachance DH, Johnson DR. Socioeconomic status and glioblastoma risk: A population-based analysis. Cancer Causes Control. 2015. 26: 179-85
40. Reames BN, Birkmeyer NJ, Dimick JB, Ghaferi AA. Socioeconomic disparities in mortality after cancer surgery: Failure to rescue. JAMA Surg. 2014. 149: 475-81
41. Robertson JT, Gunter BC, Somes GW. Racial differences in the incidence of gliomas: A retrospective study from Memphis, Tennessee. Br J Neurosurg. 2002. 16: 562-6
42. Samkange-Zeeb F, Schlehofer B, Schüz J, Schlaefer K, Berg-Beckhoff G, Wahrendorf J. Occupation and risk of glioma, meningioma and acoustic neuroma: Results from a German case-control study (interphone study group, Germany). Cancer Epidemiol. 2010. 34: 55-61
43. . SAS Institute Inc. JMP, Version 11. Cary, NC: SAS Institute Inc; 1989-2007. p.
44. Schlehofer B, Hettinger I, Ryan P, Blettner M, Preston-Martin S, Little J. Occupational risk factors for low grade and high grade glioma: Results from an international case control study of adult brain tumours. Int J Cancer. 2005. 113: 116-25
45. Schmidt LS, Nielsen H, Schmiedel S, Johansen C. Social inequality and incidence of and survival from tumours of the central nervous system in a population-based study in Denmark, 1994-2003. Eur J Cancer. 2008. 44: 2050-7
46. Sharrocks K, Spicer J, Camidge DR, Papa S. The impact of socioeconomic status on access to cancer clinical trials. Br J Cancer. 2014. 111: 1684-7
47. Sherwood PR, Dahman BA, Donovan HS, Mintz A, Given CW, Bradley CJ. Treatment disparities following the diagnosis of an astrocytoma. J Neurooncol. 2011. 101: 67-74
48. Sherwood PR, Stommel M, Murman DL, Given CW, Given BA. Primary malignant brain tumor incidence and Medicaid enrollment. Neurology. 2004. 62: 1788-93
49. Sia Y, Field K, Rosenthal M, Drummond K. Socio-demographic factors and their impact on the number of resections for patients with recurrent glioblastoma. J Clin Neurosci. 2013. 20: 1362-5
50. Simpson JR, Scott CB, Rotman M, Curran WJ, Constine LS, Fischbach AJ. Race and prognosis of brain tumor patients entering multicenter clinical trials. A report from the Radiation Therapy Oncology Group. Am J Clin Oncol. 1996. 19: 114-20
51. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005. 352: 987-96
52. Sukumar S, Ravi P, Sood A, Gervais MK, Hu JC, Kim SP. Racial disparities in operative outcomes after major cancer surgery in the United States. World J Surg. 2015. 39: 634-43
53. Teo M, Martin S, Owusu-Agyemang K, Nowicki S, Clark B, Mackinnon M. A survival analysis of GBM patients in the West of Scotland pre- and post-introduction of the Stupp regime. Br J Neurosurg. 2014. 28: 351-5
54. Tseng MY, Tseng JH, Merchant E. Comparison of effects of socioeconomic and geographic variations on survival for adults and children with glioma. J Neurosurg. 2006. 105: 297-305
55. Unger JM, Hershman DL, Albain KS, Moinpour CM, Petersen JA, Burg K. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013. 31: 536-42
56. Last cited on 2015 Apr 29. Available from: http://www.census.gov/geo/reference/.
57. Wick W, Weller M, van den Bent M, Sanson M, Weiler M, von Deimling A. MGMT testing – The challenges for biomarker-based glioma treatment. Nat Rev Neurol. 2014. 10: 372-85
58. Wigertz A, Lönn S, Hall P, Feychting M. Non-participant characteristics and the association between socioeconomic factors and brain tumour risk. J Epidemiol Community Health. 2010. 64: 736-43
59. Yi SW, Ohrr H. Agent Orange exposure and cancer incidence in Korean Vietnam veterans: A prospective cohort study. Cancer. 2014. 120: 3699-706
60. Last accessed on 2015 May 01. Available from: http://www.hrsa.gov.