- Department of Neurosurgery, Sheri-Kashmir-Institute of Medical Sciences, Kashmir, India
- Department of Microbiology, Sheri-Kashmir-Institute of Medical Sciences, Kashmir, India
Correspondence Address:
Nayil Khursheed
Department of Neurosurgery, Sheri-Kashmir-Institute of Medical Sciences, Kashmir, India
DOI:10.4103/sni.sni_105_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nayil Khursheed, Sultan Dar, Altaf Ramzan, Bashir Fomda, Nisar Humam, Wani Abrar, Sarbjit Singh, Arif Sajad, Masood Mahek, Shoaib Yawar. Spinal epidural abscess: Report on 27 cases. 26-Sep-2017;8:240
How to cite this URL: Nayil Khursheed, Sultan Dar, Altaf Ramzan, Bashir Fomda, Nisar Humam, Wani Abrar, Sarbjit Singh, Arif Sajad, Masood Mahek, Shoaib Yawar. Spinal epidural abscess: Report on 27 cases. 26-Sep-2017;8:240. Available from: http://surgicalneurologyint.com/surgicalint-articles/spinal-epidural-abscess-report-on-27-cases/
Abstract
Background:Spinal epidural abscess, although an uncommon disease, often correlates with a high morbidity owing to significant delay in diagnosis.
Methods:In a prospective 5-year study, the clinical and magnetic resonance (MR) findings, treatment protocols, microbiology, and neurological outcomes were analyzed for 27 patients with spinal epidural abscess.
Results:Patients were typically middle-aged with underlying diabetes and presented with lumbar abscesses. Those undergoing surgical intervention >36 h after the onset of symptoms had poor neurological outcomes.
Conclusion:Early recognition and timely evacuation of spinal abscesses minimized neurological morbidity and potential mortality.
Keywords: Clinical profile, epidural abscess, outcome, spinal epidural abscess
INTRODUCTION
Spinal epidural abscess (SEA) is correlated with a significant neurological morbidity attributed to direct compression and/or vascular compromise. Multiple risk factors contribute to the risk of SEA. Most SEAs are found in the thoracolumbar spine where the epidural space is large and contains more infection-prone adipose tissue.[
MATERIALS AND METHODS
Patient population: Demography
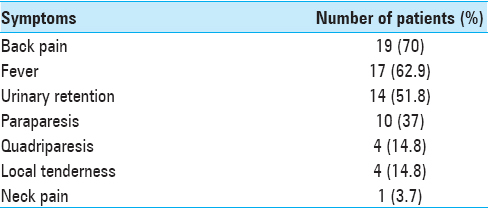
Over a 5-year period, 27 patients were diagnosed with SEA on magnetic resonance imaging (MRI). Evaluations included a detailed neurological examination, blood/other cultures, and a spinal MR. There were 14 patients <50 years of age and 13 patients >50 years. The mean age was 54 years (range 8–72 years). Back pain and fever were the most common symptoms [
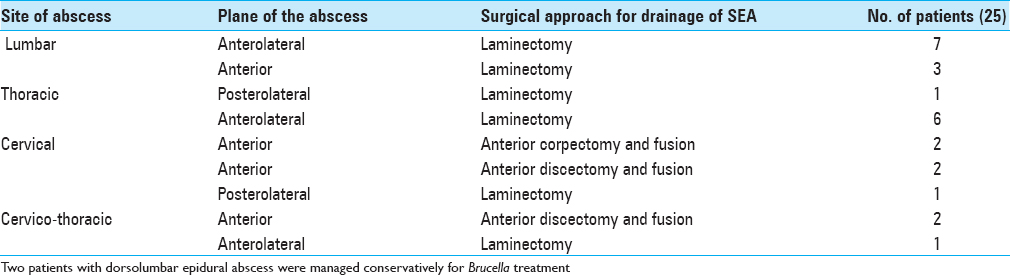
Location of abscess
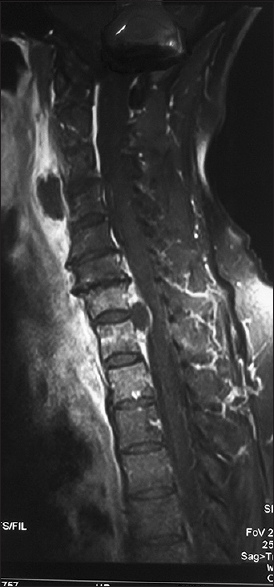
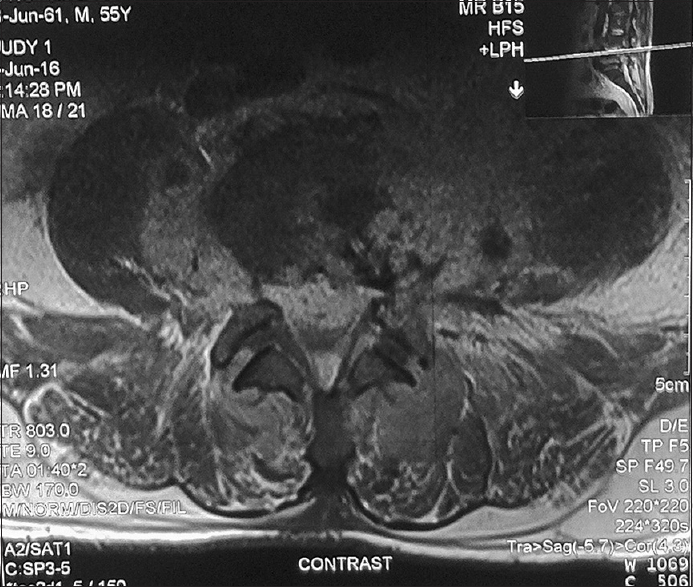
The most common location of the abscess was in the lumbar spine 37% (10/27), followed by the dorsal spine 25.9% (7/27) [Figures
Biochemical markers
Leukocytosis was found in 77.77% of our patients. We considered C-reactive protein (CRP) of >5 mg/dl as abnormal. At the beginning of the treatment, CRP levels were raised in 92.5% (25/27) of the patients.
Antibiotic management
All patients received antibiotic treatment for 6 weeks depending on culture results from the pus recovered from the spinal epidural space. In case culture was negative, patients were administered empirical antibiotic treatment.
Definition of improvement
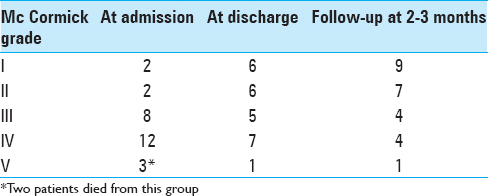
Shift in McCormick grade from poor grade (IV, V) to good grade (I–III) or increase in the McCormick grade by at least 1 grade within the good grade group and/or improvement in bladder function was considered as improvement [
Statistical analysis
All variables found to be statistically significant on univariate analysis on Fisher exact test and Students t-test were subjected to multivariate analysis using the stepwise multiple logistic regression model using the forward likelihood ratio method.
RESULTS
Treatment
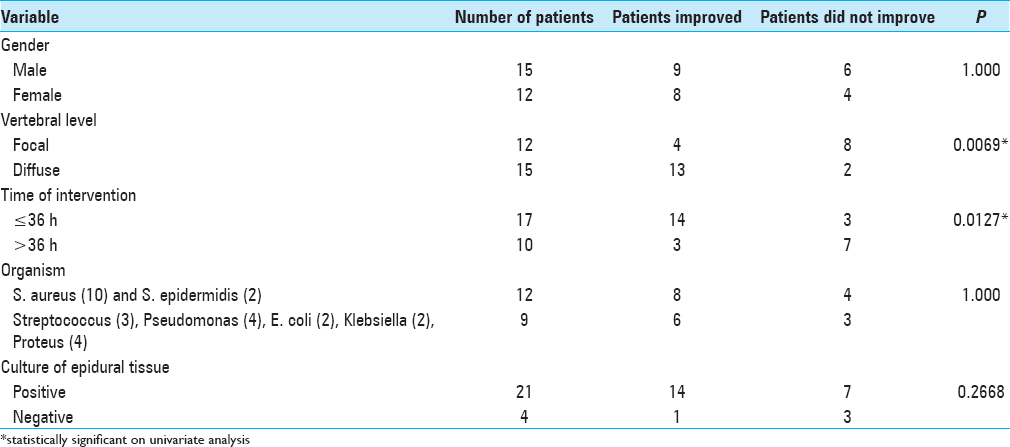
Surgery was done in 25 patients whereas 2 patients were managed conservatively for Brucellosis. We intervened within 36 h of symptoms in 62.96% (17/27) patients and after 36 h in 37.04% (10/27) patients. Antibiotics were given for a total of 6 week following surgery; intravenously for 4 weeks (this is usually not adequate for osteomyelitis; at least 6 weeks treatment or more, and must be followed with successive MR studies), and orally if possible for the next 2 weeks. In patients operated within 36 h or after 36 h of admission, neurological improvement was noted in 82.35% (14/17) of patients and 30% (3/10), respectively. The difference was statistically significant (P = 0.0127) [Tables
Microbiological spectrum
Purulent material from the SEA in 25 patients grew S. aureus in 10/25, Streptococci in 3/25, Escherichia coli, Klebsiella, and S. epidermidis in 2/25 each, and Proteus and Pseudomonas in 1/25 each; surgical cultures were negative in 4/25 patients. Blood culture grew S. aureus in 25.9% (7/27), Brucella militensis in 7.4% (2/27), and Klebsiella in 3.7 (1/27). A total of 57.14% (12/21) and 42.86% (9/21) infections were caused by Staphylococcus and other bacteria, respectively.
Clinical outcome
Clinical outcome was assessed at the time of discharge and 2–3 months later; 17 patients (62.9%) had a good functional grade at discharge, which increased to 74.07% (20/27) 2–3 months later. There were 2 deaths in the hospital due to sepsis, associated comorbidities, and poor response to treatment leading to multiorgan failure and death [
DISCUSSION
Early diagnosis and adequate treatment are known to be important for the management of patients with SEA, however, despite early diagnosis and adequate treatment, only 45% of the patients recover fully from this devastating spinal infectious disease.[
The classic triad of back pain, fever, and neurological deficits may only be found in only a minority of patients with SEA.[
A delay in surgery for SEA typically correlates with poor neurological outcomes. In our study, it was found that patients operated within 36 h of onset had better outcome than those operated after 36 h. We found that 82.35% (14/17) of our patients improved neurologically when surgery was done within 36 h of symptom onset as compared to 30% (3/10) improvement when it was done after 36 h. Similar results have also been seen in other studies.[
In contrast, medical therapy alone may be favored in patients with panspinal SEA involvement or those with complete paresis for 72 h or more or when surgery is deemed too risky.[
At the same time, predictive factors for failure of medical management also have been published which include diabetes, CRP level >115 mg/L, positive blood cultures, age >65 years, and methicillin-resistant S. aureus.[
Indices of infection available for use in the diagnosis and management of SEA include white blood cell (WBC) count, erythrocyte sedimentation rate (ESR), and CRP level. ESR is a more sensitive screening test than the leukocyte count. The CRP level should also be determined in suspected or confirmed cases of SEA. The CRP level increases faster at the onset of inflammation and returns to normal sooner than the ESR.[
Given that most cases of SEA originate from a source away from the vertebral canal, an effort must be made to identify the causative organism and the primary site of infection. Blood culture provides isolation of the causative pathogen in approximately 60% of the patients and remains negative in 40%; however, in our study it was found to be positive only in 44.44% (12/27). One must screen for other potential causes of bacteremia.
Antibiotics should be administered to cover Staphylococci (including MRSA), Streptococci, and Gram-negative pathogens such as a combination of vancomycin with either piperacillin-tazobactam or a third-or fourth-generation cephalosporin is often recommended.[
CONCLUSION
Most patients with bacterial SEA are not diagnosed in time. A high level of suspicion in patients with back pain and fever may help in early diagnosis. Contrast MRI is the investigation of choice. Timely surgical evacuation of the abscess provides the bacterial yield in most of the patients and reverses the neurodeficit.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Akalan N, Ozgen T. Infection as a cause of spinal cord compression: A review of 36 spinal epidural abscess cases. Acta Neurochir (Wien). 2000. 142: 17-23
2. Baker AS, Ojemann RG, Swartz MN, Richardson EP. Spinal epidural abscess. N Engl J Med. 1975. 293: 463-8
3. Darouiche RO, Hamill RJ, Greenberg SB, Weathers SW, Musher DM. Bacterial spinal epidural abscess. Review of 43 cases and literature survey. Medicine. 1992. 71: 369-85
4. Epstein NE. Timing and prognosis of surgery for spinal epidural abscess: A review. Surg Neurol Int. 2015. 6: S475-86
5. Jensen AG, Espersen F, Skinhoi P, Rosdahl VT, Frimodt-Moller N. Increasing frequency of vertebral osteomyelitis following Staphylococcus aureus bacteraemia in Denmark 1980–1990. J Infect. 1997. 34: 113-8
6. Kaufman DM, Kaplan JG, Litman N. Infectious agents in spinal epidural abscesses. Neurology. 1980. 30: 844-50
7. Pathak A, Singh P, Gehlot P, Dhaneria M. Spinal epidural abscess treated with antibiotics alone. BMJ Case Rep. 2013. 30: bcr2013009285-
8. Reihsaus E, Waldbaur H, Seeling W. Spinal Epidural abscess: A meta-analysis of 915 patients. Neurosurg Rev. 2000. 23: 175-204
9. Rigamonti D, Liem L, Sampath P, Knoller N, Namaguchi Y, Schreibman DL. Spinal epidural abscess: Contemporary trends in etiology, evaluation, and management. Surg Neurol. 1999. 52: 189-96
10. Sendi P, Bregenzer T, Zimmerli W. Spinal epidural abscess in clinical practice. QJM. 2008. 101: 1-12
11. Tompkins M, Panuncialman I, Lucas P, Palumbo M. Spinal epidural abscess. J Emerg Med. 2010. 39: 384-90