- Department of Neurosurgery, NTT Medical Center Tokyo, Shinagawa, Tokyo, Japan.
DOI:10.25259/SNI_385_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sho Tsunoda, Tomohiro Inoue, Kazuaki Naemura, Atsuya Akabane. Surgical removal using V3-radial artery graft-V4 bypass and occipital artery-posterior inferior cerebellar artery bypass for a giant thrombosed aneurysm of vertebral artery compressing brain stem: Case report. 15-Nov-2019;10:220
How to cite this URL: Sho Tsunoda, Tomohiro Inoue, Kazuaki Naemura, Atsuya Akabane. Surgical removal using V3-radial artery graft-V4 bypass and occipital artery-posterior inferior cerebellar artery bypass for a giant thrombosed aneurysm of vertebral artery compressing brain stem: Case report. 15-Nov-2019;10:220. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=9756
Abstract
Background: Giant thrombosed vertebral artery aneurysms (GTVAs) are difficult disease to treat. Here, we are reporting a case of GTVA successfully treated with excluding the pathological segment and restoring the anterograde blood flow of the parent artery, highlighting the reliable surgical procedure.
Case Description: A 55-year-old man with a left GTVA complained of right hemiparesis (manual muscle testing 4/5) represented by hand clumsiness and gait disturbance, in addition to severe left-sided dysesthesia, was referred to our hospital. The posterior inferior cerebellar artery (PICA) was incorporated into the GTVA segment, and the contralateral vertebral artery showed atherosclerotic change. Thus, we decided to treat the aneurysm with aneurysm trapping and thrombectomy, in conjunction with V3-radial artery graft (RAG)-V4 bypass and occipital artery (OA)-PICA bypass through a suboccipital transcondylar approach. The distal end of the dilated segment was meandering and deflecting outwardly to the vicinity of the internal auditory canal and was stretched in an axial direction. Thus, the V4 stump can be transposed to the triangle space made by the medulla, lower cranial nerves, and sigmoid sinus, and we could perform a safe and reliable anastomosis through the corridor. After the surgery, the compression of the brain stem was released, and right hemiparesis was improved completely after rehabilitation. The patient was discharged with a modified Rankin Scale score of 1.
Conclusion: Trapping of the aneurysm and thrombectomy are the most radical treatment for GTVA, and if possible, reconstruction of anterograde blood flow with V3-RAG-V4 bypass and OA-PICA bypass is desirable.
Keywords: Giant thrombosed aneurysm, Occipital artery-posterior inferior cerebellar artery bypass, Reconstruction, V3-radial artery graft-V4 bypass
INTRODUCTION
Giant thrombosed vertebral artery aneurysms (GTVAs) are associated with a very poor prognosis once they become symptomatic.[
Herein, we present a case with GTVA involving the posterior inferior cerebellar artery (PICA), who was treated with trapping of the aneurysm and thrombectomy, in conjunction with a V3-radial artery graft (RAG)-V4 bypass and occipital artery (OA)-PICA bypass. Furthermore, we discuss the safety and reliability of the surgical procedure in detail.
CASE DESCRIPTION
A 55-year-old man with a left GTVA was referred to our institution for possible surgical treatment. On admission, neurological examinations revealed right hemiparesis (manual muscle testing 4/5) represented by hand clumsiness and gait disturbance, in addition to severe left-sided dysesthesia. These symptoms had progressively worsening over the previous month.
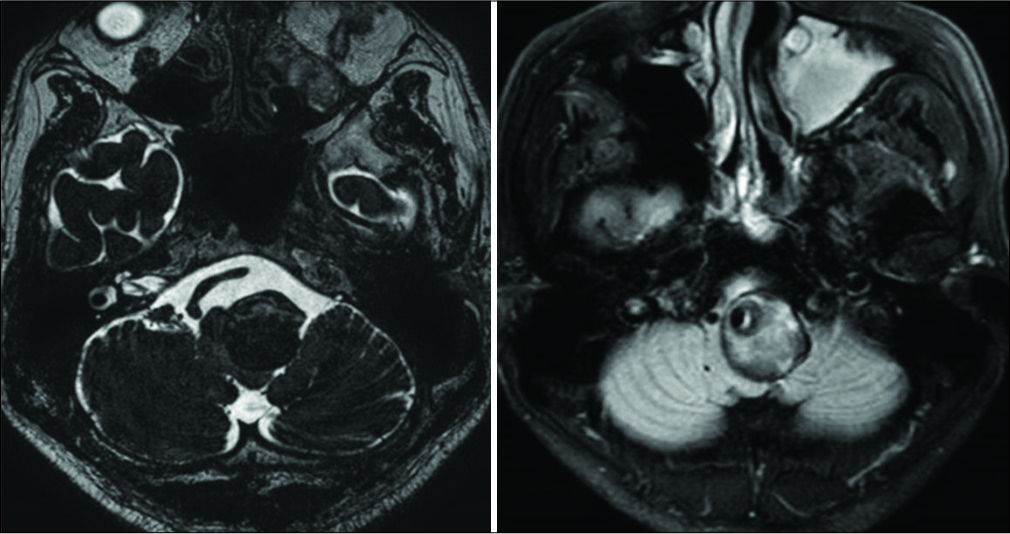
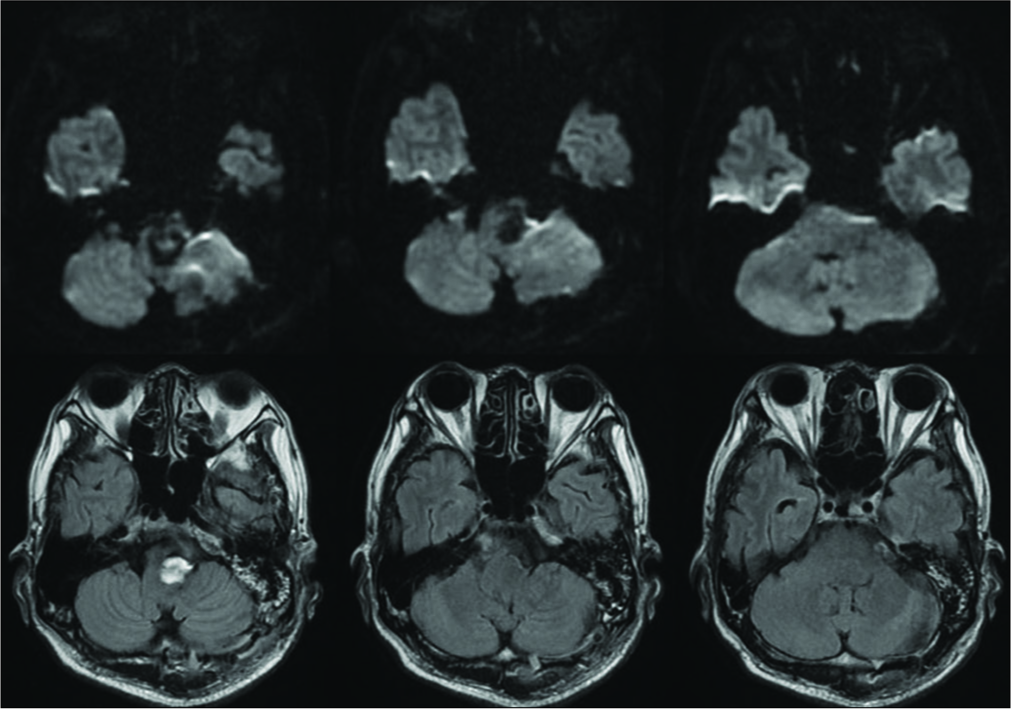
Magnetic resonance imaging (MRI) revealed a GTVA at the left V4 portion (maximum diameter of 30 mm) that severely compressed the medulla oblongata contralaterally. The GTVA also demonstrated a heterogenic signal intensity suggesting different stages of thrombus formation inside the aneurysm [
Figure 2:
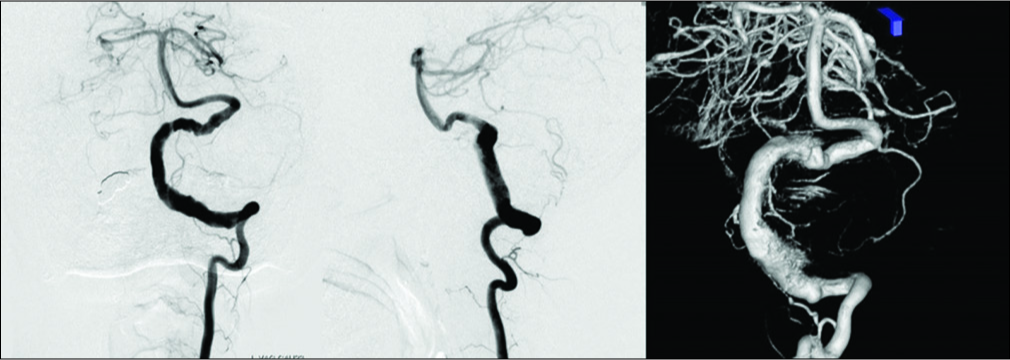
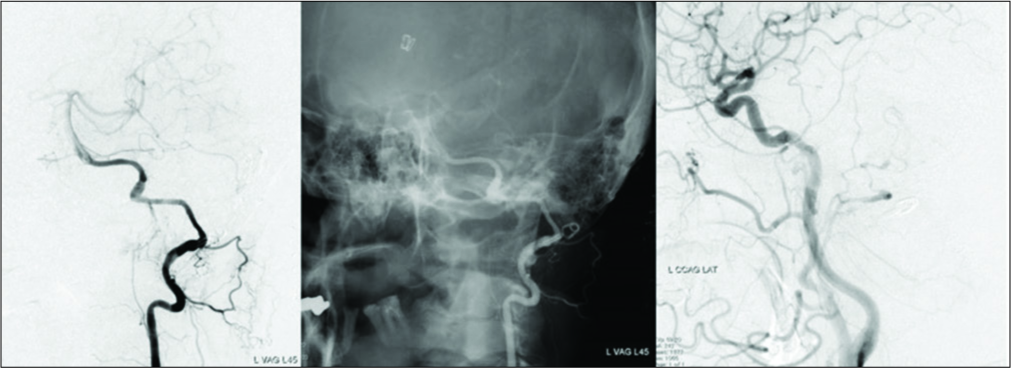
Preoperative digital subtraction angiography showing serpentine aneurysm of the left vertebral artery with normal appearance distal V4 segment which was pushed up distally and thus bending toward the internal auditory canal. The posterior inferior cerebellar artery was possibly incorporated in the lesioned part and retrogradely opacified through the ipsilateral superior cerebellar artery and its pial anastomosis.
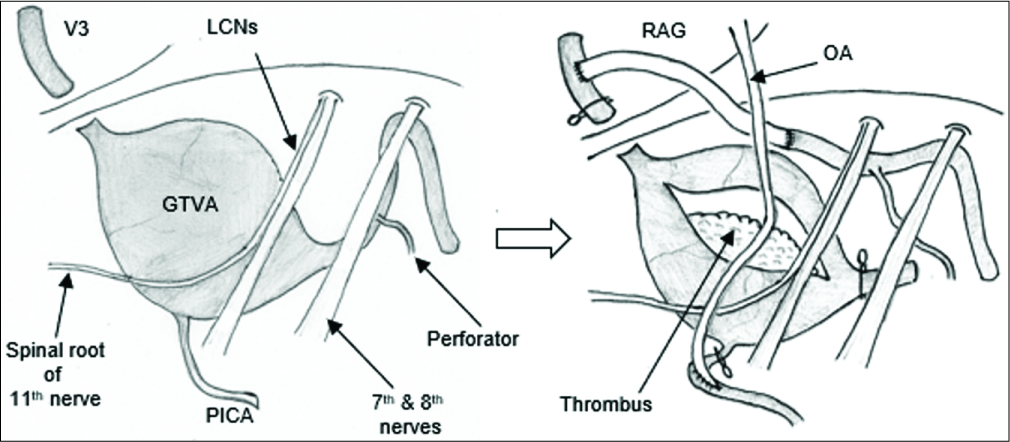
After a thorough discussion, trapping of the GTVA and intraluminal thrombectomy, followed by V3-RAG-V4 bypass reconstruction, in conjunction with possible OA-PICA bypass, was indicated [
The surgical procedure was performed with motor evoked potentials, somatosensory evoked potentials, and auditory brainstem response monitoring. The patient placed in a park bench position with the left side up, and his head was slightly rotated contralaterally to subluxate the craniocervical joint so that the left condylar fossa was well exposed for far lateral drilling. An L-shape skin incision was performed, and the skin flap was reflected medially [
Figure 5:
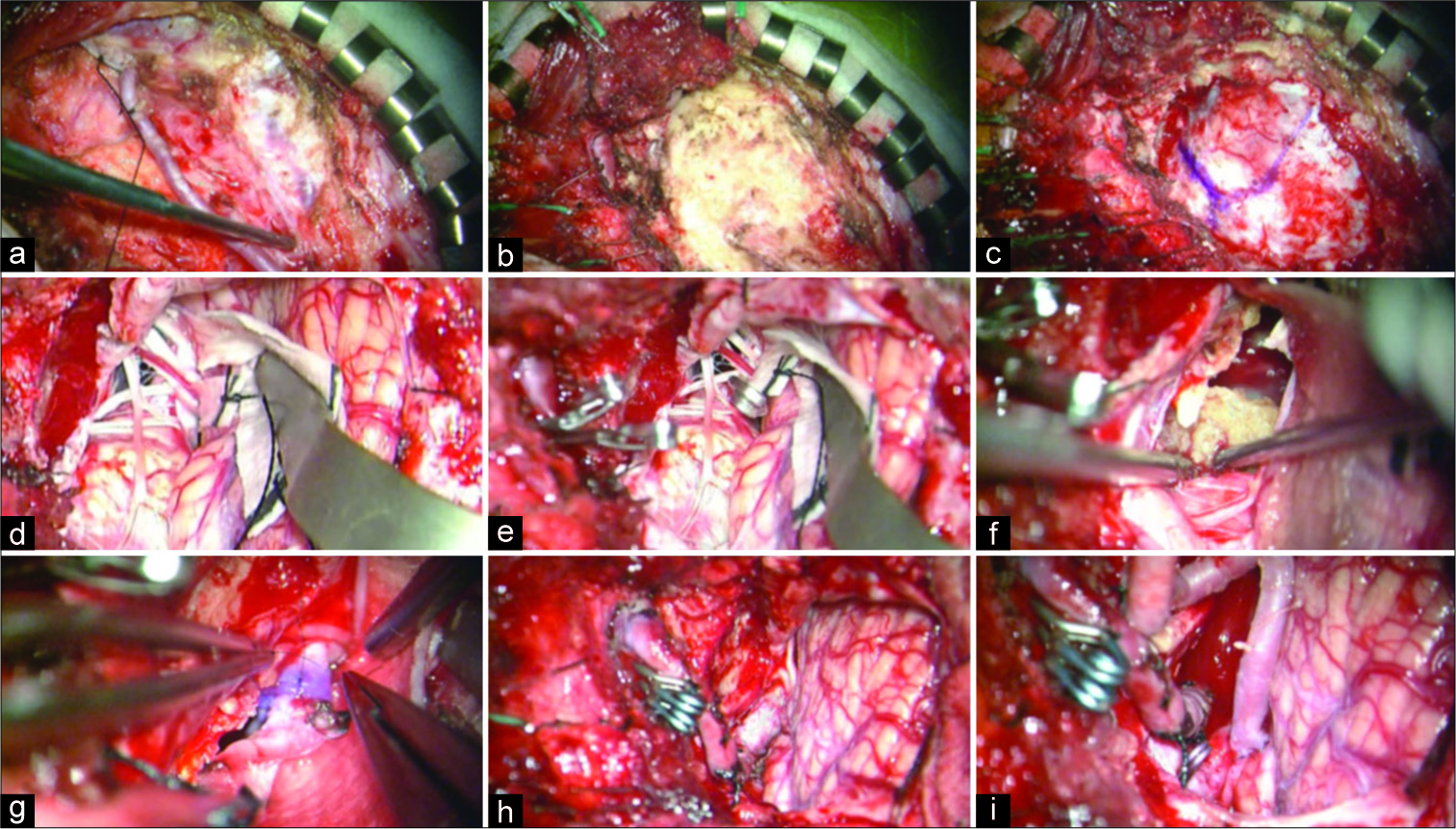
The intraoperative images. (a) The occipital artery (OA) was harvested from the exit of the digastric groove to the entry to the skin. (b) V3 portion of the left vertebral artery was exposed in the suboccipital triangle. (c) Left suboccipital craniotomy and far-lateral drilling were performed. (d) The giant thrombosed vertebral artery aneurysm (GTVA) compressing the medulla oblongata as well as the spinal root of the 11th nerve medially was exposed. (e) The GTVA was trapped between V3 portion and just distal to the dilated segment. (f) Intraluminal laminated thrombus was removed. (g and h) V3-radial artery graft-V4 bypass was performed through the space lateral to the 11th nerve. (i) Finally, OA-posterior inferior cerebellar artery anastomosis was added.
Postoperative MRI and DSA demonstrated the disappearance of the aneurysm, improvement of brainstem compression, and good patency of each bypass [
DISCUSSION
The mechanism underlying intra-aneurysmal thrombosis and aneurysm enlargement was reported to involve repeated minor hemorrhages from the vasa vasorum during the dynamic process of neovascularization,[
In recent years, endovascular procedures have been considered less invasive, especially for avoiding effects on the LCNs, when compared with open surgical manipulation.[
Reconstruction of the VA after trapping and thrombectomy is not typically performed for a patent contralateral VA, as the procedure is invasive and complicated. However, there are several reasons why revascularization of VA should be considered whenever possible. First, there is no guarantee of permanent patency of the contralateral VA because of severe atherosclerosis in our patient. Indeed, most patients with VA aneurysms were reported to have accompanying atherosclerotic degeneration in other vessels.[
There are two important points related to obtaining a surgical corridor to perform a safe RAG-distal V4 anastomosis in GTVA cases. First, a suboccipital transcondylar approach with complete drilling of the condylar fossa can be used to widen the triangular space made by a spinal root of the 11th nerve, LCNs, and medulla. However, in the present case, the huge GTVA deviated the spinal root of the 11th nerve rostromedially. Thus, we performed the anastomosis through the space lateral to the medially deviated 11th nerve/medulla, inferior to the LCNs, and medial to the sigmoid sinus. Second, in cases of serpentine VA aneurysms, the distal end of the dilated segment is often meandering and deflecting outwardly to the vicinity of the internal auditory canal and is stretched in an axial direction.[
CONCLUSION
Trapping of the aneurysm and thrombectomy, in conjunction with V3-RAG-V4 bypass and OA-PICA bypass, should be considered as the most radical treatment for GTVA involving the PICA.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on:
www.surgicalneurologyint.com
Acknowledgments
We thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
References
1. Ahn JY, Han IB, Kim TG, Yoon PH, Lee YJ, Lee BH. Endovascular treatment of intracranial vertebral artery dissections with stent placement or stent-assisted coiling. AJNR Am J Neuroradiol. 2006. 27: 1514-20
2. Chang SD, Marks MP, Steinberg GK. Recanalization and rupture of a giant vertebral artery aneurysm after hunterian ligation: Case report. Neurosurgery. 1999. 44: 1117-20
3. Fang YB, Wen WL, Yang PF, Zhou Y, Wu YN, Hong B. Long-term outcome of tubridge flow diverter(S) in treating large vertebral artery dissecting aneurysms a pilot study. Clin Neuroradiol. 2017. 27: 345-50
4. He M, Zhang H, Lei D, Mao BY, You C, Xie XD. Application of covered stent grafts for intracranial vertebral artery dissecting aneurysms. J Neurosurg. 2009. 110: 418-26
5. Iihara K, Murao K, Sakai N, Soeda A, Ishibashi-Ueda H, Yutani C. Continued growth of and increased symptoms from a thrombosed giant aneurysm of the vertebral artery after complete endovascular occlusion and trapping: The role of vasa vasorum. Case report. J Neurosurg. 2003. 98: 407-13
6. Inoue TTamura ASaito I. Video 1. Available from: https://thejns.org/view/journals/neurosurg-focus/38/videosuppl1/2015.v1.focus14465.xml [Last accessed on 30 Apr 2019].
7. Iwai T, Naito I, Shimaguchi H, Suzuki T, Tomizawa S. Angiographic findings and clinical significance of the anterior and posterior spinal arteries in therapeutic parent artery occlusion for vertebral artery aneurysms. Interv Neuroradiol. 2000. 6: 299-309
8. Kado K, Hirai S, Kobayashi S, Kobayashi E, Yamakami I, Uchino Y. Potential role of the anterior spinal artery in preventing propagation of thrombus in a therapeutically occluded vertebral artery: Angiographic studies before and after endovascular treatment. Neuroradiology. 2002. 44: 347-54
9. Katsuno M, Mizunari T, Kobayashi S, Takahashi H, Teramoto A. Rupture of a vertebral artery dissecting aneurysm developing immediately after trapping of a dissecting aneurysm on the contralateral vertebral artery: Case report. Neurol Med Chir (Tokyo). 2009. 49: 468-70
10. Krings T, Alvarez H, Reinacher P, Ozanne A, Baccin CE, Gandolfo C. Growth and rupture mechanism of partially thrombosed aneurysms. Interv Neuroradiol. 2007. 13: 117-26
11. Mahmood A, Dujovny M, Torche M, Dragovic L, Ausman JI. Microvascular anatomy of foramen caecum medullae oblongatae. J Neurosurg. 1991. 75: 299-304
12. Matsushima T, Kawashima M, Masuoka J, Mineta T, Inoue T. Transcondylar fossa (supracondylar transjugular tubercle) approach: Anatomic basis for the approach, surgical procedures, and surgical experience. Skull Base. 2010. 20: 83-91
13. Mizutani T, Aruga T, Kirino T, Miki Y, Saito I, Tsuchida T. Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery. 1995. 36: 905-11
14. Nagahiro S, Takada A, Goto S, Kai Y, Ushio Y. Thrombosed growing giant aneurysms of the vertebral artery: Growth mechanism and management. J Neurosurg. 1995. 82: 796-801
15. Nakatomi H, Segawa H, Kurata A, Shiokawa Y, Nagata K, Kamiyama H. Clinicopathological study of intracranial fusiform and dolichoectatic aneurysms: Insight on the mechanism of growth. Stroke. 2000. 31: 896-900
16. Nashimoto T, Komata T, Honma J, Yamashita S, Seki Y, Kurashima A. Successful treatment of bilateral vertebral artery dissecting aneurysms with subarachnoid hemorrhage: Report of three cases. J Stroke Cerebrovasc Dis. 2012. 21: 422-7
17. Nijensohn DE, Saez RJ, Reagan TJ. Clinical significance of basilar artery aneurysms. Neurology. 1974. 24: 301-5
18. Ota N, Tanikawa R, Eda H, Matsumoto T, Miyazaki T, Matsukawa H. Radical treatment for bilateral vertebral artery dissecting aneurysms by reconstruction of the vertebral artery. J Neurosurg. 2016. 125: 953-63
19. Park JC, Kwon BJ, Cho YD, Han MH. Growing thrombosed dissecting aneurysm of the vertebral artery after endovascular proximal artery occlusion: The role of the vasa vasorum. Neurointervention. 2009. 4: 33-7
20. Sharma BS, Gupta A, Ahmad FU, Suri A, Mehta VS. Surgical management of giant intracranial aneurysms. Clin Neurol Neurosurg. 2008. 110: 674-81
21. Siddiqui AH, Abla AA, Kan P, Dumont TM, Jahshan S, Britz GW. Panacea or problem: Flow diverters in the treatment of symptomatic large or giant fusiform vertebrobasilar aneurysms. J Neurosurg. 2012. 116: 1258-66