- Department of Neurosurgery, Houston Methodist Hospital, Houston, Texas, United States
Correspondence Address:
Jonathan J. Lee
Department of Neurosurgery, Houston Methodist Hospital, Houston, Texas, United States
DOI:10.25259/SNI-125-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jonathan J. Lee, Bradley Daniels, Ryan J. Austerman, Brian D. Dalm. Symptomatic, left-sided deep brain stimulation lead edema 6 h after bilateral subthalamic nucleus lead placement. 24-Apr-2019;10:68
How to cite this URL: Jonathan J. Lee, Bradley Daniels, Ryan J. Austerman, Brian D. Dalm. Symptomatic, left-sided deep brain stimulation lead edema 6 h after bilateral subthalamic nucleus lead placement. 24-Apr-2019;10:68. Available from: http://surgicalneurologyint.com/surgicalint-articles/9286/
Abstract
Background:Deep brain stimulation (DBS) lead edema can be a serious, although rare, complication in the postoperative period. Of the few cases that have been reported, the range of presentation has been 33 h–120 days after surgery.
Case Description:We report a case of a 75-year-old male with a history of Parkinson’s disease who underwent bilateral placement of subthalamic nucleus DBS leads that resulted in symptomatic, left-sided lead edema 6 h after surgery, which is the earliest reported case.
Conclusions:DBS lead edema is noted to be a self-limiting phenomenon. It is critical to recognize the possibility of lead edema as a cause of postoperative encephalopathy even in the acute phase after surgery. Although it is important to rule out other causes of postoperative changes in the patient examination, the recognition of lead edema can help to avoid extraneous diagnostic tests or DBS lead revision or removal.
Keywords: Deep brain stimulation, edema, lead
INTRODUCTION
Deep brain stimulation (DBS) is a well-established treatment option that is used for medically refractory forms of a variety of neurological disorders.[
CASE REPORT
A 75-year-old male with a 10-year history of Parkinson’s disease progressively refractory to rasagiline and carbidopa-levodopa use was evaluated in our neurosurgery clinic. At the initial evaluation, the patient complained of tremors in both upper extremities, worse on the right than the left. The patient had no significant comorbidities, and the patient had passed all preoperative neuropsychological testing. After a previous evaluation by his neurologist, the patient was referred for bilateral subthalamic nucleus (STN) DBS lead placement. A review of a preexisting brain subsequent magnetic resonance imaging (MRI) demonstrated no abnormal findings other than small vessel ischemic changes. Diagnostic studies did not show any contraindications to DBS lead placement.
Operative details
A Leksell Stereotactic frame was mounted on the patient’s head. An O-arm® was used to perform a stereotactic volumetric computed tomography (CT) scan of the head. These CT images were then merged with a Stealth navigation system protocol MRI of the brain. A three-dimensional reconstruction of the images was performed to identify the locations of the anterior commissure (AC) and posterior commissure (PC). The coordinates chosen were 12 mm to the left and right of the AC-PC midpoint, 3 mm behind the AC-PC midpoint, and 4 mm below the AC-PC midpoint. The AC-PC distance was measured to be 26.87 mm. After a bifrontal incision, bilateral burr holes overlying the coronal suture were created approximately 4 cm from the midline at each side. Next, a microelectrode drive for the right side was mounted to the Leksell frame. Microelectrode recordings (MERs) were not optimal for the right side, so an O-arm CT scan was performed which showed that the electrodes were lateral and anterior to the desired target. After making appropriate adjustments, MERs were retaken. The center trajectory was used as the tract for the DBS electrode for the right side. Medtronic DBS leads (model 3387S-40) were used for both targets. The electrode was then lowered to the target on the right side and the patient exhibited improvement of his left-sided symptoms. The Leksell arc was then reconfigured for the left side coordinates and the microelectrode drive was remounted. For the left side, the electrode in the lateral tract was used which was 0.5 mm below target. The electrode was lowered to the target and the patient exhibited improvement of his right-sided upper extremity tremor. There were no intraoperative complications.
Postoperative course
In the recovery room, 6 h after the operation, the patient’s wife noted that the patient did not seem like himself. Per nursing report, the patient only had limited responses when spoken to.
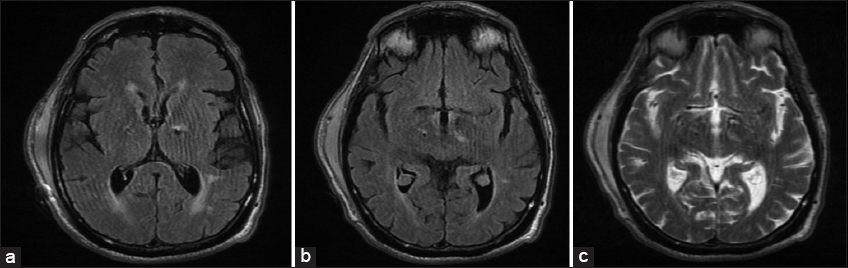
The patient was able to say his name, was moving all four of his extremities, but would not follow commands. Overall, there were only mild decreases in the patient’s tremors from baseline. The patient exhibited normal vitals and remained hemodynamically stable. A CT scan of the head showed the adequate placement of the DBS electrodes at the bilateral STNs, mild extra-axial pneumocephalus along the frontal convexities, and no other intracranial abnormalities. Scheduled IV dexamethasone 6 mg every 6 h was administered for predicted cerebral edema. A MRI of the brain [
Figure 1
(a and b) T2-FLAIR magnetic resonance imagings of the brain showing increased FLAIR signal along the left deep brain stimulation (DBS) electrode at the level of the left thalamus and supra-adjacent white matter. (c) T2-weighted MRI of the brain (same axial cut as
On the postoperative day 1, the patient was noted to have limited speech and was following commands in all four extremities. He continued to be on scheduled IV dexamethasone. On the postoperative day 3, the patient’s IV dexamethasone was discontinued and he was started on tapered PO methylprednisolone. On the postoperative day 6, a repeat CT of the head showed postsurgical changes and no evidence of acute intracranial abnormality. The patient’s neurologic examination was improved. The patient was discharged to an acute rehabilitation center on this same day.
DISCUSSION
In itself, DBS lead edema is a rare complication of DBS surgery; in addition, we also present the earliest recorded evidence – 6 h postoperatively – of symptomatic DBS lead edema after surgery. Deogaonkar et al.[
Unilateral lead edema is seen more frequently than bilateral edema.[
Perhaps, location of the DBS lead plays a role in the formation of postoperative lead edema. In three single case reports of lead edema, all were cases of STN lead placement for Parkinson’s disease that experienced symptomatic lead edema.[
In terms of treatment options, we believe that imaging is the first step whenever a postoperative patient who underwent DBS lead placement presents with neurologic deficits. A CT of the head should first be performed to rule out an acute hemorrhage. The CT should then be examined for proper positioning of the leads. If the CT is adequate and all medical (e.g., chemical, metabolic, etc.) abnormalities have been ruled out, we believe that an MRI of the brain without contrast should then be performed. We also utilize a short dose of IV decadron in patients in whom we suspect lead edema. If lead edema is on the top of the differential after imaging studies, we believe that this is a self-limiting process and can be managed conservatively without revisions of the DBS leads. The patient should be examined in the hospital with serial neurologic examinations.
CONCLUSIONS
Here, we present an unusual case of rapid-onset lead edema merely 6 h postoperatively in a 75-year-old male undergoing bilateral STN DBS placement for Parkinson’s disease. DBS lead edema should be on the differential for any postoperative DBS patient experiencing symptomatic change in neurologic status. Lead edema is a poorly understood complication of DBS surgery and many hypotheses exist. Although most reported cases occur days after surgery, we report a case only 6 h postoperatively. We believe that treatment options for lead edema rely on conservative management with initial CT and MRI imaging. Recognition of lead edema can avoid unnecessary removal or revision of DBS leads.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understand that her name and initial will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Deogaonkar M, Nazzaro JM, Machado A, Rezai A. Transient, symptomatic, post-operative, non-infectious hypodensity around the deep brain stimulation (DBS) electrode. J Clin Neurosci. 2011. 18: 910-5
2. Englot DJ, Glastonbury CM, Larson PS. Abnormal T2-weighted MRI signal surrounding leads in a subset of deep brain stimulation patients. Stereotact Funct Neurosurg. 2011. 89: 311-7
3. Fenoy AJ, Simpson RK. Risks of common complications in deep brain stimulation surgery:Management and avoidance. J Neurosurg. 2014. 120: 132-9
4. Gerard CS, Metman LV, Pal G, Karl J, Sani S. Severe, symptomatic, self-limited unilateral DBS lead edema following bilateral subthalamic nucleus implantation:Case report and review of the literature. Neurologist. 2016. 21: 58-60
5. Lefaucheur R, Derrey S, Borden A, Wallon D, Ozkul O, Gérardin E. Post-operative edema surrounding the electrode:An unusual complication of deep brain stimulation. Brain Stimul. 2013. 6: 459-60
6. Morishita T, Okun MS, Burdick A, Jacobson CE, Foote KD. Cerebral venous infarction:A potentially avoidable complication of deep brain stimulation surgery. Neuromodulation. 2013. 16: 407-13
7. Schoen NB, Jermakowicz WJ, Luca CC, Jagid JR. Acute symptomatic peri-lead edema 33 hours after deep brain stimulation surgery:A case report. J Med Case Rep. 2017. 11: 103-