- Department of Radiology and Imaging Sciences, Division of Neurointerventional Radiology, Salt Lake City, Utah, USA
- Ochsner Clinical School, University of Queensland, Brisbane, Australia
- Department of Radiology and Biomedical Imaging, Division of Neurointerventional Radiology, University of California San Francisco, San Francisco, California, USA
Correspondence Address:
Daniel L. Cooke
Department of Radiology and Biomedical Imaging, Division of Neurointerventional Radiology, University of California San Francisco, San Francisco, California, USA
DOI:10.4103/sni.sni_255_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Matthew D. Alexander, Jeffrey M. Rebhun, Steven W. Hetts, Matthew R. Amans, Fabio Settecase, Robert J. Darflinger, Christopher F. Dowd, Van V. Halbach, Randall T. Higashida, Daniel L. Cooke. Technical factors affecting outcomes following endovascular treatment of posterior circulation atherosclerotic lesions. 20-Nov-2017;8:284
How to cite this URL: Matthew D. Alexander, Jeffrey M. Rebhun, Steven W. Hetts, Matthew R. Amans, Fabio Settecase, Robert J. Darflinger, Christopher F. Dowd, Van V. Halbach, Randall T. Higashida, Daniel L. Cooke. Technical factors affecting outcomes following endovascular treatment of posterior circulation atherosclerotic lesions. 20-Nov-2017;8:284. Available from: http://surgicalneurologyint.com/surgicalint-articles/technical-factors-affecting-outcomes-following-endovascular-treatment-of-posterior-circulation-atherosclerotic-lesions/
Abstract
Background:Atherosclerotic disease of the vertebrobasilar system causes significant morbidity and mortality. All lesions require aggressive medical management, but the role of endovascular interventions remains unsettled. This study examines such endovascular interventions for vertebrobasilar atherosclerosis.
Methods:Retrospective review was performed of prospectively maintained procedure logs at three hospitals with comprehensive neurointerventional services. Patients with angiographically-proven stenosis undergoing elective stent placement were selected for analysis of demographic factors, lesion characteristics, and treatment details. Multivariate analysis was performed to evaluate for associations with ischemic stroke, death, and functional status as measured by modified Rankin scale at multiple intervals.
Results:One hundred and twenty-three lesions were treated in 110 patients. A total of 43 (58.1%) lesions caused stroke, while 66 (89.2%) caused transient ischemic attacks (TIAs). Forty lesions (32.5%) were at the vertebral origin; 97 (60.2%) were intracranial. A total of 112 (91.1%) were treated successfully. 4 (3.3%) of 10 (8.1%) procedural complications were symptomatic. Intracranial lesions were associated with death at 1 and 2 years (OR 24.91, P 2 at last contact (OR 12.83, P P = 0.046) and mRS >2 at last contact (OR 0.234, P = 0.018) when angioplasty was performed with a device other than that packaged with the stent.
Conclusion:Endovascular treatment of vertebrobasilar atherosclerosis can be performed safely, particularly for vertebral origin lesions. Higher rates of technical failure and complication may be acceptable for certain intracranial lesions due to their refractory nature and the morbidity caused by such lesions. Treatment should be tailored to features of each individual lesion.
Keywords: Angioplasty, atherosclerosis, ischemic stroke, stenting
INTRODUCTION
Atherosclerosis of the vertebrobasilar system accounts for a significant portion of ischemic strokes. The optimal role for endovascular therapies remains uncertain, particularly with respect to intracranial disease, in light of poorer outcomes of the stenting cohort in the SAMMPRIS trial.[
MATERIALS AND METHODS
Under IRB-approved protocols, medical records were retrospectively reviewed by searching prospectively maintained procedure databases at a large academic medical center and two affiliated hospitals, all with high volume comprehensive neurointerventional services. All patients with stenosis of the vertebral or basilar arteries were identified. From this group, patients undergoing elective angioplasty or stent deployment were selected. Patients with luminal narrowing due to disease processes other than atherosclerosis were excluded. Patients in whom an intervention was attempted but unsuccessful were included in an intention to treat analysis.
Information was gathered according to the guidelines of the Standards Committee of the Society for NeuroInterventional Surgery for investigations of endovascular treatment of intracranial atherosclerotic disease.[
Timing and type of clinical and imaging follow up were determined by the primary interventionalist; no uniform protocol existed between practitioners. The most recent date of contact was determined for long-term follow up. For those patients with available records, the Social Security death index was queried to screen for deaths among patients lost to follow up.[
RESULTS
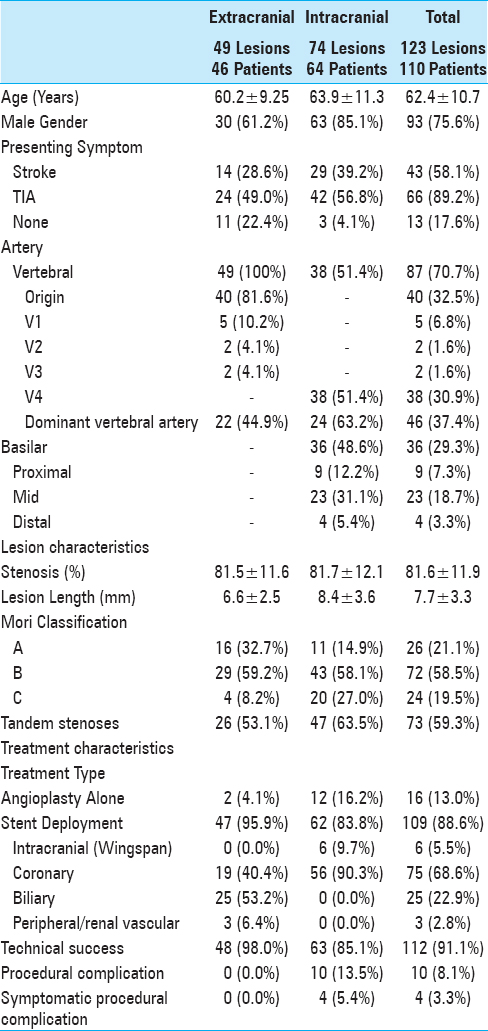
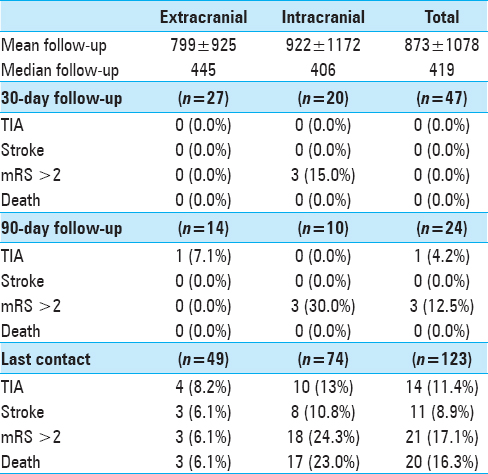
One hundred and twenty-three lesions in 110 patients were treated between August 1998 and August 2013 and met inclusion criteria. Patient demographics, lesion characteristics, and treatment features are summarized in
Results of univariate analysis are summarized in Supplemental Tables
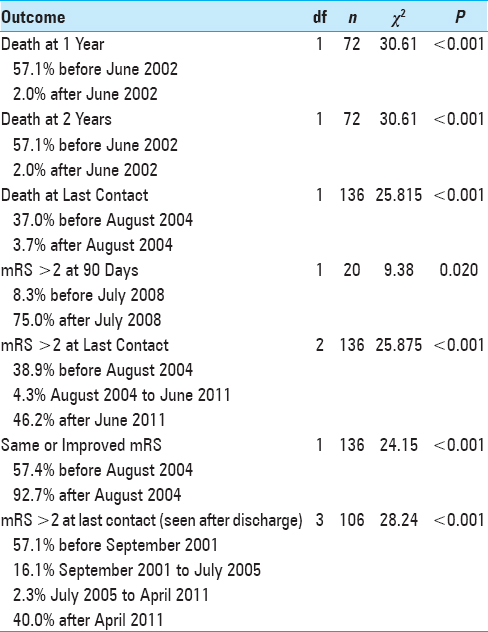
Temporal inflection points reflecting changes in outcomes identified by recursive partitioning are summarized in
In multivariate analysis, statistical significance persisted for the association of intracranial lesion location with death at 1 year (OR, 24.91; 95% CI, 2.746–226.0; P < 0.001), death at 2 years (OR, 24.91; 95% CI, 2.746–226.0; P < 0.001), and mRS >2 at last contact (OR, 12.83, 95% CI, 2.567–641.0; P < 0.001). When stent deployment was performed, statistically significant inverse relationships were noted between use of an angioplasty balloon other than that packaged with a stent with death at last contact (OR, 0.303; 95% CI, 0.094–0.979; P = 0.046) and mRS>2 at last contact (OR, 0.234; 95% CI 0.070–0.780; P = 0.018).
DISCUSSION
In the United States, intracranial atherosclerosis causes 10–15% of ischemic strokes, and is the etiology of up to half of stroke in populations outside of the U.S.[
Twenty-five to forty percent of ischemic strokes are in the posterior circulation.[
Surgical treatments for posterior circulation atherosclerosis have shown no benefit or are prohibitively morbid.[
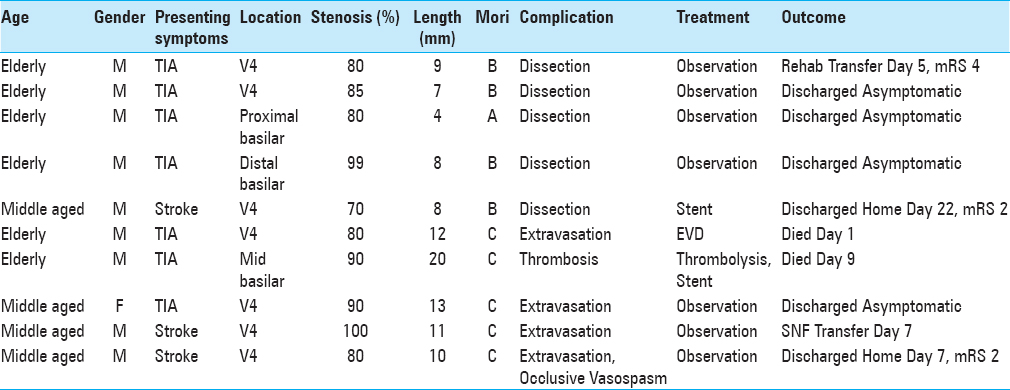
Technical failure was associated with poor outcomes regardless the stent type (balloon-mounted vs. self-expanding, biliary vs. coronary vs. intracranial). Additionally, the need to consider treatment of these lesions on a case-by-case basis is reflected in the multiple device types operators preferred over many years. Attempts to simplify and generalize devices belie the importance of planning each treatment individually to best fit lesion characteristics. This is suggested by the improved outcomes when using an angioplasty balloon other than that packaged with a stent, a statistically significant relationship that persisted in multivariate analysis.
In addition to selecting the proper devices, understanding the inherent risks of different lesions is important. Intracranial lesion location was a strong predictor of poor outcomes, with statistical significance in the multivariate models for association with death at one year and two years, as well as mRS>2 at last contact. Success rates were lower for these lesions compared to extracranial disease (85.1% vs. 98.0%, respectively), and all procedural complications in the current analysis occurred during treatment of intracranial lesions. Additionally, presence of tandem stenoses was predictive of adverse events in univariate analysis. Such outcomes, which are concordant with findings elsewhere, should be taken into account when considering endovascular treatment of intracranial posterior circulation atherosclerosis.[
Whereas endovascular treatment of intracranial lesions carries inherent risks, such treatment of extracranial disease, particularly at the vertebral artery origin, is relatively safe. Prior studies have demonstrated high rates of technical success and few procedural complications.[
Endovascular device technology continues to advance, as does medical management. This study found that better outcomes occurred following publication of the SPARCL trial, after which time statin treatment for cervicocerebral atherosclerosis became standard at our medical center. Indeed, we have previously reported the beneficial impact of statin treatment on our cohort of patients treated with angioplasty or stenting.[
Given the above findings and discussion, endovascular treatment of atherosclerosis in the posterior circulation can be achieved with high levels of technical success and good outcomes. However, further investigation is needed considering limitations of this current study, most of which are due to retrospective design and selection bias inherent in studying only patients for whom treatment was elected. Lack of prospectively developed follow-up protocols limited data capture within early post-procedure periods and the similarly limited assessment of follow up imaging. Additionally, this study reflects over sixteen years of interventions and includes patients treated with methods formerly considered appropriate but not currently standard of care. As such, adverse technical events might be lower for interventions performed with contemporary techniques and equipment.
CONCLUSION
Endovascular treatment of atherosclerosis of the vertebrobasilar system can be performed with high rates of technical success and few complications. This is particularly true for lesions of the extracranial vertebral arteries, for which endovascular treatment should be sought for lesions refractory to medical management. Higher rates of failure and complication may be acceptable for intracranial lesions refractory to medical care due to the poor natural history prognosis of such lesions and the morbidity inherent to infarctions in this territory.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abuzinadah AR, Alanazy MH, Almekhlafi MA, Duan Y, Zhu H, Mazighi M. Stroke recurrence rates among patients with symptomatic intracranial vertebrobasilar stenoses: Systematic review and meta-analysis. J Neurointerv Surg. 2016. 8: 112-6
2. Alexander M, Cooke D, Meyers P, Amans M, Dowd C, Halbach V. Lesion stability characteristics outperform degree of stenosis in predicting outcomes following stenting for symptomatic intracranial atherosclerosis. J Neurointerv Surg. 2016. 8: 19-23
3. Alexander MD, Meyers PM, English JD, Stradford TR, Sung S, Smith WS. Symptom Differences and Pretreatment Asymptomatic Interval Affect Outcomes of Stenting for Intracranial Atherosclerotic Disease. AJNR Am J Neuroradiol. 2014. 35: 1157-62
4. Alexander MD, Rebhun JM, Hetts SW, Kim AS, Nelson J, Kim H. Lesion location, stability, and pretreatment management: Factors affecting outcomes of endovascular treatment for vertebrobasilar atherosclerosis. JNeurointerv Surg. 2016. 8: 466-70
5. Amin-Hanjani S, Rose-Finnell L, Richardson D, Ruland S, Pandey D, Thulborn KR. Vertebrobasilar Flow Evaluation and Risk of Transient Ischaemic Attack and Stroke study (VERiTAS): Rationale and design. Int J Stroke. 2010. 5: 499-505
6. Applegate RJ, Sacrinty MT, Kutcher MA, Santos RM, Gandhi SK, Little WC. Effect of length and diameter of drug-eluting stents versus bare-metal stents on late outcomes. Circ Cardiovasc Interv. 2009. 2: 35-42
7. Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991. 337: 1521-6
8. Barakate MS, Snook KL, Harrington TJ, Sorby W, Pik J, Morgan MK. Angioplasty and stenting in the posterior cerebral circulation. J Endovasc Ther. 2001. 8: 558-65
9. Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: Analysis of 1,000 consecutive patients with first stroke. Stroke. 1988. 19: 1083-92
10. Caplan LR, Gorelick PB, Hier DB. Race, sex and occlusive cerebrovascular disease: A review. Stroke. 1986. 17: 648-55
11. Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998. 55: 1475-82
12. Chastain HD, Campbell MS, Iyer S, Roubin GS, Vitek J, Mathur A. Extracranial vertebral artery stent placement: In-hospital and follow-up results. J Neurosurg. 1999. 91: 547-52
13. Chaturvedi S, Turan TN, Lynn MJ, Kasner SE, Romano J, Cotsonis G. Risk factor status and vascular events in patients with symptomatic intracranial stenosis. Neurology. 2007. 69: 2063-8
14. Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. NEngl J Med. 2005. 352: 1305-16
15. Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF. Stenting versus aggressive medical therapy for intracranial arterial stenosis. NEngl J Med. 2011. 365: 993-1003
16. Cloud GC, Crawley F, Clifton A, McCabe DJ, Brown MM, Markus HS. Vertebral artery origin angioplasty and primary stenting: Safety and restenosis rates in a prospective series. J Neurol Neurosurg Psychiatry. 2003. 74: 586-90
17. .editorsArmonk, NY: IBM Corp; p.
18. Crawley F, Brown MM. Percutaneous transluminal angioplasty and stenting for vertebral artery stenosis. Cochrane Database Syst Rev. 2000. p. CD000516-
19. Fiorella D, Chow MM, Anderson M, Woo H, Rasmussen PA, Masaryk TJ. A 7-year experience with balloon-mounted coronary stents for the treatment of symptomatic vertebrobasilar intracranial atheromatous disease. Neurosurgery. 2007. 61: 236-42
20. Fiorella D, Levy EI, Turk AS, Albuquerque FC, Niemann DB, Aagaard-Kienitz B. US multicenter experience with the wingspan stent system for the treatment of intracranial atheromatous disease: Periprocedural results. Stroke. 2007. 38: 881-7
21. Gomez CR, Misra VK, Liu MW, Wadlington VR, Terry JB, Tulyapronchote R. Elective stenting of symptomatic basilar artery stenosis. Stroke. 2000. 31: 95-9
22. Groschel K, Schnaudigel S, Pilgram SM, Wasser K, Kastrup A. A systematic review on outcome after stenting for intracranial atherosclerosis. Stroke. 2009. 40: e340-7
23. Gulli G, Khan S, Markus HS. Vertebrobasilar stenosis predicts high early recurrent stroke risk in posterior circulation stroke and TIA. Stroke. 2009. 40: 2732-7
24. Gupta R, Al-Ali F, Thomas AJ, Horowitz MB, Barrow T, Vora NA. Safety, feasibility, and short-term follow-up of drug-eluting stent placement in the intracranial and extracranial circulation. Stroke. 2006. 37: 2562-6
25. Hass WK, Fields WS, North RR, Kircheff , II , Chase NE, Bauer RB. Joint study of extracranial arterial occlusion. II. Arteriography, techniques, sites, and complications. JAMA. 1968. 203: 961-8
26. Hatano T, Tsukahara T, Miyakoshi A, Arai D, Yamaguchi S, Murakami M. Stent placement for atherosclerotic stenosis of the vertebral artery ostium: Angiographic and clinical outcomes in 117 consecutive patients. Neurosurgery. 2011. 68: 108-16
27. Huang YN, Gao S, Li SW, Huang Y, Li JF, Wong KS, Kay R. Vascular lesions in Chinese patients with transient ischemic attacks. Neurology. 1997. 48: 524-5
28. Hussain MS, Fraser JF, Abruzzo T, Blackham KA, Bulsara KR, Derdeyn CP. Standard of practice: Endovascular treatment of intracranial atherosclerosis. J Neurointerv Surg. 2012. 4: 397-406
29. Imparato AM. Vertebral arterial reconstruction: A nineteen-year experience. J Vasc Surg. 1985. 2: 626-34
30. Jenkins JS, White CJ, Ramee SR, Collins TJ, Chilakamarri VK, McKinley KL. Vertebral artery stenting. Catheter Cardiovasc Interv. 2001. 54: 1-5
31. Jenkins JS, Patel SN, White CJ, Collins TJ, Reilly JP, McMullan PW. Endovascular stenting for vertebral artery stenosis. J Am Coll Cardiol. 2010. 55: 538-42
32. Jiang WJ, Cheng-Ching E, Abou-Chebl A, Zaidat OO, Jovin TG, Kalia J. Multicenter analysis of stenting in symptomatic intracranial atherosclerosis. Neurosurgery. 2012. 70: 25-30
33. Karameshev A, Schroth G, Mordasini P, Gralla J, Brekenfeld C, Arnold M. Long-term outcome of symptomatic severe ostial vertebral artery stenosis (OVAS). Neuroradiology. 2010. 52: 371-9
34. Malek AM, Higashida RT, Phatouros CC, Lempert TE, Meyers PM, Gress DR. Treatment of posterior circulation ischemia with extracranial percutaneous balloon angioplasty and stent placement. Stroke. 1999. 30: 2073-85
35. Marks MP, Marcellus ML, Do HM, Schraedley-Desmond PK, Steinberg GK, Tong DC. Intracranial angioplasty without stenting for symptomatic atherosclerotic stenosis: Long-term follow-up. AJNR Am J Neuroradiol. 2005. 26: 525-30
36. Marks MP, Wojak JC, Al-Ali F, Jayaraman M, Marcellus ML, Connors JJ. Angioplasty for symptomatic intracranial stenosis: Clinical outcome. Stroke. 2006. 37: 1016-20
37. Marquardt L, Kuker W, Chandratheva A, Geraghty O, Rothwell PM. Incidence and prognosis of > or = 50% symptomatic vertebral or basilar artery stenosis: Prospective population-based study. Brain. 2009. 132: 982-8
38. Mori T, Fukuoka M, Kazita K, Mori K. Follow-up study after intracranial percutaneous transluminal cerebral balloon angioplasty. AJNR Am J Neuroradiol. 1998. 19: 1525-33
39. Moufarrij NA, Little JR, Furlan AJ, Leatherman JR, Williams GW. Basilar and distal vertebral artery stenosis: Long-term follow-up. Stroke. 1986. 17: 938-42
40. Nahab F, Lynn MJ, Kasner SE, Alexander MJ, Klucznik R, Zaidat OO. Risk factors associated with major cerebrovascular complications after intracranial stenting. Neurology. 2009. 72: 2014-9
41. Nakazawa G, Finn AV, Joner M, Ladich E, Kutys R, Mont EK. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: An autopsy study. Circulation. 2008. 118: 1138-45
42. Phatouros CC, Lefler JE, Higashida RT, Meyers PM, Malek AM, Dowd CF. Primary stenting for high-grade basilar artery stenosis. AJNR Am J Neuroradiol. 2000. 21: 1744-9
43. Piotin M, Spelle L, Martin JB, Weill A, Rancurel G, Ross IB. Percutaneous transluminal angioplasty and stenting of the proximal vertebral artery for symptomatic stenosis. AJNR Am J Neuroradiol. 2000. 21: 727-31
44. Quinn J, Kramer N, McDermott D. Validation of the Social Security Death Index (SSDI): An Important Readily-Available Outcomes Database for Researchers. West J Emerg Med. 2008. 9: 6-8
45. Qureshi AI, Ziai WC, Yahia AM, Mohammad Y, Sen S, Agarwal P. Stroke-free survival and its determinants in patients with symptomatic vertebrobasilar stenosis: A multicenter study. Neurosurgery. 2003. 52: 1033-9
46. Rohde S, Seckinger J, Hahnel S, Ringleb PA, Bendszus M, Hartmann M. Stent design lowers angiographic but not clinical adverse events in stenting of symptomatic intracranial stenosis - results of a single center study with 100 consecutive patients. Int J Stroke. 2013. 8: 87-94
47. Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995. 26: 14-20
48. Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000. 21: 643-6
49. Shofti R, Tio F, Beyar R. Neointimal vascularization and intimal thickening in response to self-expanding stents: A swine model. Int J Cardiovasc Intervent. 2004. 6: 61-7
50. Spetzler RF, Hadley MN, Martin NA, Hopkins LN, Carter LP, Budny J. Vertebrobasilar insufficiency. Part 1: Microsurgical treatment of extracranial vertebrobasilar disease. J Neurosurg. 1987. 66: 648-61
51. Vajda Z, Miloslavski E, Guthe T, Fischer S, Albes G, Heuschmid A. Treatment of stenoses of vertebral artery origin using short drug-eluting coronary stents: Improved follow-up results. AJNR Am J Neuroradiol. 2009. 30: 1653-6
52. Whisnant JP, Cartlidge NE, Elveback LR. Carotid and vertebral-basilar transient ischemic attacks: Effect of anticoagulants, hypertension, and cardiac disorders on survival and stroke occurrence--a population study. Ann Neurol. 1978. 3: 107-15
53. Wong KS, Huang YN, Gao S, Lam WW, Chan YL, Kay R. Intracranial stenosis in Chinese patients with acute stroke. Neurology. 1998. 50: 812-3
54. Wong KS, Li H, Chan YL, Ahuja A, Lam WW, Wong A. Use of transcranial Doppler ultrasound to predict outcome in patients with intracranial large-artery occlusive disease. Stroke. 2000. 31: 2641-7
55. Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. 2006. 1: 158-9
56. Zaidat OO, Klucznik R, Alexander MJ, Chaloupka J, Lutsep H, Barnwell S. The NIH registry on use of the Wingspan stent for symptomatic 70-99% intracranial arterial stenosis. Neurology. 2008. 70: 1518-24