- Department of Neurosurgery, School of Medicine, Universitas Pembangunan Nasional “Veteran” Jakarta, Indonesia.
- Department of Neurosurgery, Cipto Mangunkusumo Hospital, School of Medicine, University of Indonesia, Jakarta, Indonesia.
Correspondence Address:
Feda Anisah Makkiyah
Department of Neurosurgery, Cipto Mangunkusumo Hospital, School of Medicine, University of Indonesia, Jakarta, Indonesia.
DOI:10.25259/SNI_62_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Feda Anisah Makkiyah, Wismaji Sadewo. Technical report: Simple method of animal stroke model of luminal occlusion of middle cerebral artery in Indonesia. 19-Jul-2019;10:143
How to cite this URL: Feda Anisah Makkiyah, Wismaji Sadewo. Technical report: Simple method of animal stroke model of luminal occlusion of middle cerebral artery in Indonesia. 19-Jul-2019;10:143. Available from: http://surgicalneurologyint.com/surgicalint-articles/9515/
Abstract
Background: Although there are many experimental studies describing the suture method of middle cerebral artery occlusion (MCAO) in rats, this is still a new procedure in Indonesia and the techniques for applying this stroke model in animal research are not well known. There has been a perception in Indonesian scientific community that the technique would be difficult and require advanced equipment. The aim of this study is to demonstrate that it is possible to perform the technique with minimal resources using simple method and basic surgical loupe equipment.
Methods: A total of 30 male Wistar rats, aged 6 months, weighing 250 g–400 g Wistar rats, were obtained from the Bandung Biofarma Pasteur. Preliminary trials were performed to gain an understanding of the detailed anatomy of the animals and to master the techniques. An ×8 loupe magnification was used for all surgical steps in this study except taking of surgical operation photos. The procedures applied simple methods, using two loop temporary knots instead of any animal vascular clips.
Results: After an extensive training period, two of the 30 rats died within 4 weeks after the procedure. The effects of MCAO were confirmed clinically and by hematoxylin-eosin staining pathology slides.
Conclusion: With simple methods, this MCAO procedure could be implemented in developing countries such as Indonesia without the requirement for advanced equipment.
Keywords: Middle cerebral artery occlusion, Simple methods, Surgical loupe, Temporary knot, Wistar rat
INTRODUCTION
Stroke ischemia is a still a huge burden in Indonesia. The World Health Organization Country Health Profiles 2012 show that stroke is the number one causes of death in Indonesia with a prevalence of 21%.[
However, applying this stroke ischemia model in rats is still difficult in Indonesia. Many Indonesian researchers lack experience in the surgical techniques required of this model and are reluctant or feel that they are unable to perform the procedure because it requires advanced equipment such as aneurysmal animal clips and a laboratory surgical microscope. This study aims to describe step by step the surgical techniques of luminal occlusion of the middle cerebral artery with some modification of temporary knots as a substitute for clips, and using a surgical loupe that is more affordable than a laboratory surgical microscope. The techniques described are considered to be highly suitable for implementation in Indonesia which has a serious shortage of advanced equipment in many areas. It is hoped that application of this technique using basic, affordable equipment will lead to an increase in the number of patients being successfully treated for stroke ischemia in developing countries, especially in Indonesia.
MATERIALS AND METHODS
Animals
All surgical procedures were performed in accordance with institutional guidelines and conducted under 8 times surgical loupe magnification. A total of 30 male Wistar rats, aged 6 months, weighing 250–400 g, were taken from the Bandung Biofarma Pasteur and held in half-day dark and half day bright cages at a temperature of 22–24°C and with food and water ad libitum. The animals were not fasted before the surgery. The animals were anesthetized by 10 mg/kg i Ket-A-Xyl (Ketamine hydrochloride) 100 mg, xylazine (hydrochloride) 20 mg, atropine sulfate (monohydrate) 1 mg, excipients q.s. ad 1 mL) Perros, Ecuador given intraperitoneally. The animals were not intubated and blood gases were not monitored during the middle cerebral artery occlusion (MCAO). The Ethics Commission of the Faculty of Medicine University of Indonesia gave permission to perform the described experiments.
After a week adaptation period in the Animal Research Facility of the School of Medicine of University of Indonesia, the rats were operated on two neurosurgeons and two residents trained and experienced in MCAO techniques.
Suture preparation
Nylon 4.0 sutures were cut to a length 20 mm. One end of the suture was heated to form a smooth, spherical shape which was observed under a surgical loupe. The suture was sterilized with alcohol and stored in a heparin saline solution [
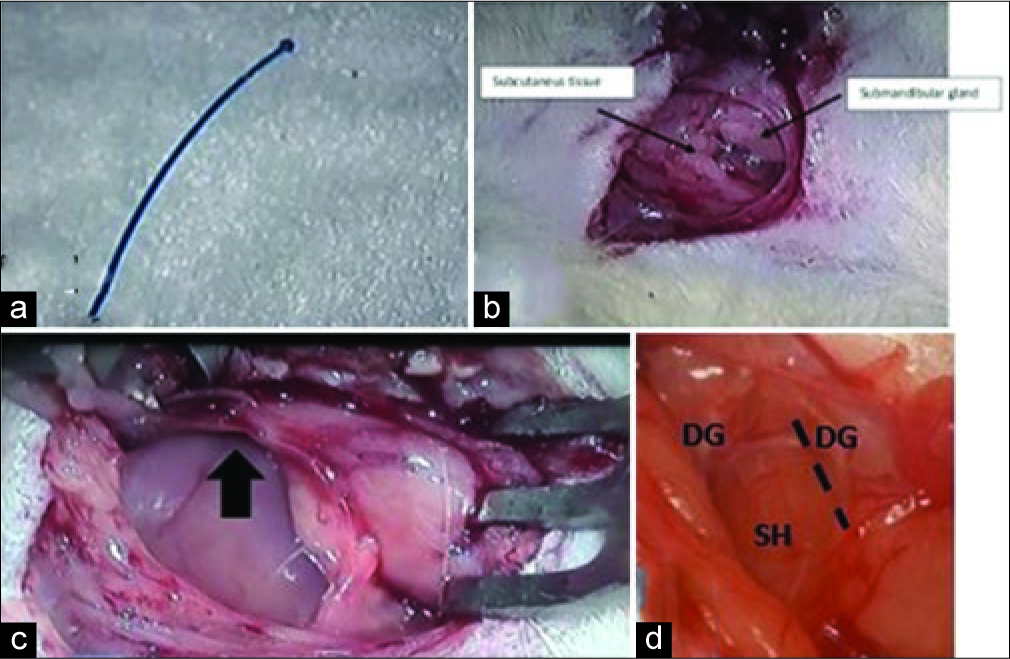
Figure 1:
Preparation (a) 20 mm nylon 4.0 length to be inserted to the lumen of internal carotid artery, (b) After dissection of subcutaneous tissue, bring the submandibular gland to the lateral. (c) After inserting the retractor, bring the submandibular gland to the lateral position, the dissection goes to the rostrolateral (black arrow). (d) Establish a triangle to form as a corridor to differentiating the right and left sides (DG=Digastric muscle, SH=Sternohyoid muscle). Using this corridor, the next dissection is made between the DG and SH (black dash line).
Surgical techniques
We used a modification of surgical procedures originally described by Koizumi[
Under the surgical loupe, a longitudinal cervical midline incision was made (approximately 2.0 cm) through subcutaneous tissue and platysma muscle [
The second landmark is the distal part of the digastric muscle and the sternohyoid muscle. We performed a dissection in between them. Here, the ECA and Communis Carotid Artery (CCA) are clearly apparent [
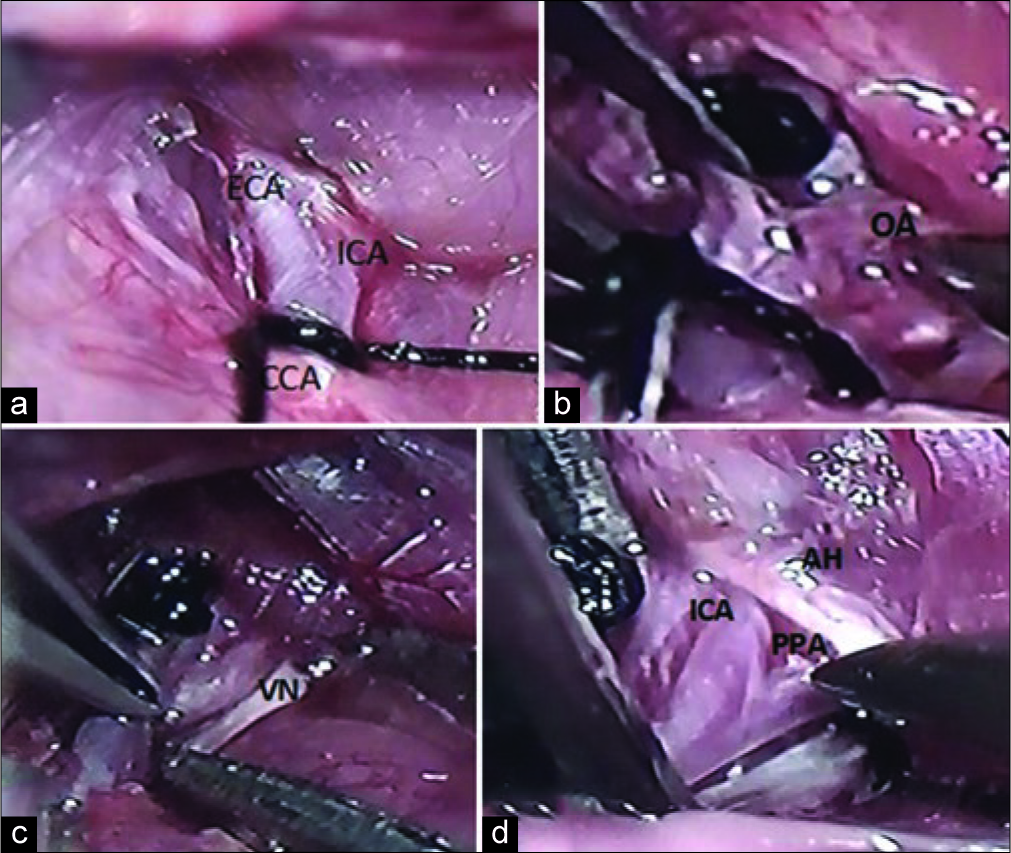
Figure 2:
Position of the double knot. (a) The first knot was made in communis carotid artery. (b) The ophthalmic artery is one of the branches of external carotid artery that goes to the lateral. (c) The vagal nerve was below the internal carotid artery. (d) The internal carotid artery was isolated and carefully separated from the lateral adjacent vagus nerve. The ansa hypoglossal was overlying pterygopalatine artery and internal carotid artery. The 4.0 silk suture was inserted between pterygopalatine artery and an internal carotid artery (blue arrow) is shown direction of suture inserted to make a knot around pterygopalatine artery.
Two double knot modifications
The first knot was made in the CCA with a double loop. The knot will be removed after the occlusion. Two double loop knots were considered adequate to reduce the blood flow and consequently reduce the bleeding from the arteriotomy. The first branch of ECA, artery of thyroidea superior went to the medial and ophthalmic artery (OA) that went to the lateral [
Meticulous dissection of the perivascular structures (fascia, adventitia, and sympathetic plexus) was performed around ECA using the sharp curved forceps. The double loop 4.0 silk suture was applied to the ECA (second knot).
After securing the blood flow from the CCA and ECA, the next step is to make the third knot around the internal carotid artery (ICA).
A dissection was made to the lateral side to find the ICA that lies on top of the vagus nerve [
Herein, we made the third double knot on the ICA that was closed to the bifurcation. Looking to the rostral, we see extracranial branch of ICA and the pterygopalatine artery (PPA) [
At this point, we found that it is necessary sometimes to pull upward the hyoid cartilage to note the bifurcation of ICA.
The important last step was to knot the PPA to ensure that the inserted thread was directed to distal ICA. We performed the new maneuver to make a permanent knot (fourth knot) around the PPA. First, we dissected the fat tissue below the bifurcation between PPA and ICA. Ansa hypoglossal could be seen overlying between PPA and ICA [
This step provided a clear bifurcation between the ICA and PPA. Here, we located the 4.0 silk suture from the medial part of ICA and came out in the gap between the ICA and PPA. One end of the suture was now in the lateral part of the ICA. After this step, we took the other end of the suture (that was still in the lateral part of the ICA) with the curve forceps from underneath the ICA. Moreover, now, the suture was in the lateral side of the PPA [
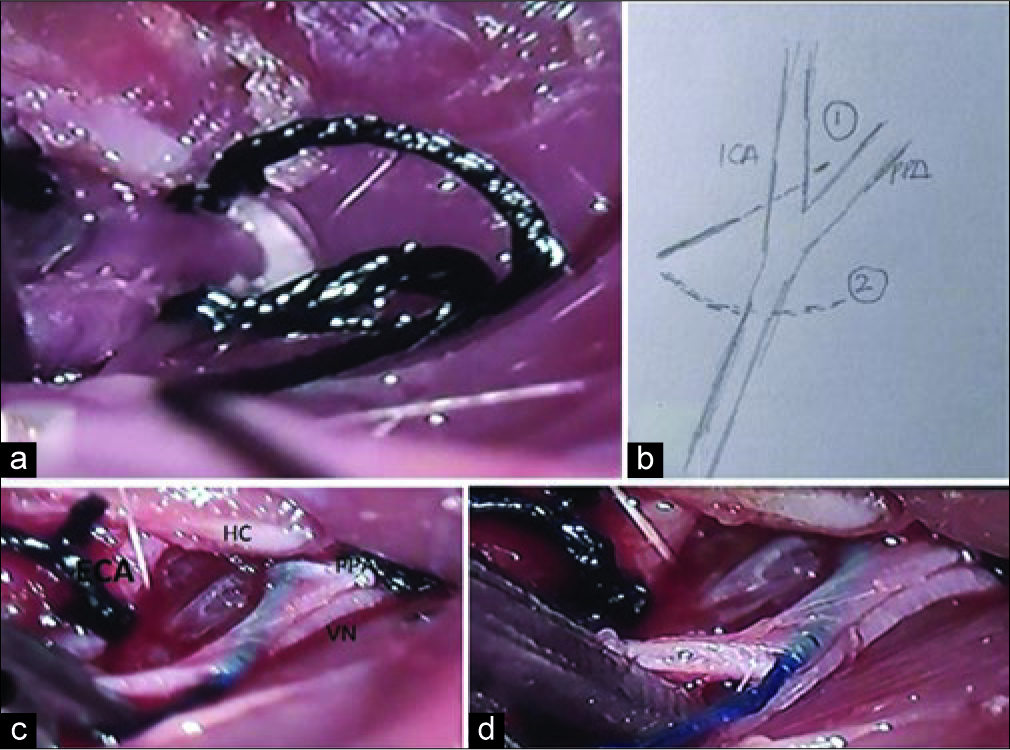
Figure 3:
Steps in making double knot. (a) Step to put a knot in pterygopalatine artery. (b) The pathway to knot pterygopalatine artery. (c) The permanent knot of the pterygopalatine artery. (d) Nylon suture was inserted from internal carotid artery. Pterygopalatine artery was shown with silk suture (has been tied) and external carotid artery has been looped by silk.
Pads were not put under the neck because we found that this caused hyperextension of the trachea which resulted in some rats dying during the procedure.
The final step was to insert the 4.0 silk suture into the ICA. Beforehand, we coagulated the proximal side of a 2 cm – 4.0 nylon with diathermy forceps. After knotting the PPA, we then made a double knot of silk suture in the ICA. The arteriotomy was made with 1 cc syringe needle in between the two temporary knots of the ICA (with a double knot around ICA). The nylon was inserted 15–18 mm until mild resistance felt and left about 0.2–0.5 cm outside [
The finishing steps. We injected 5 cc normal saline, 1 cc paracetamol, and 1 cc ceftriaxone intraperitoneally. The knots of silk suture around the ECA, CCA, and ICA near bifurcation were released. Skin was sutured with 4.0 silk suture and the wound was given Kemicetine skin ointment.
The time of occlusion was recorded and set for 90 min. At 80 min, rats were neurologically tested and given intraperitoneally Ketamine 0.05 cc diluted with 0.2 cc normal saline. After the occlusion time was finished (90 min), the incision of the neck was opened and the 4.0 nylon was pulled out 10 mm. We observed the bleeding around the arteriotomy and when the operation site was clean from the bleed, the skin was closed with 4.0 silk suture.
The rats were returned to a warm cage. Every day rats were observed for any difficulties in eating and drinking. When any problems were observed, water was given by an orogastric tube.
RESULTS
Learning experience and potential pitfalls
Because this is a new technique in Indonesia, we performed two preliminary experiments. In the first 30 Wistar male rats were operated, we were able to perform the procedures successfully on six rats, two were not successful, and 22 rats died (two intraoperative, five due to excessive anesthesia, and 15 due to subarachnoid hemorrhage (SAH). The second preliminary trials were conducted with 12 rats, of which two were not successful and 10 died due to SAH.
These trials did not use the techniques described in this paper. In these two preliminary trials, we coagulated all the branches of the ECA because it provided wide space for luminal 4.0 nylon insertion. We thought this coagulation of the branches of the ECA would require a longer recovery time. We also thought that the cause of SAH was the length of the suture (20 mm in length) that we inserted from the left ICA. It was revealed in the postmortem examination of these animals that the cause of death was probably the perforation of the ACA beyond the ostium of the left MCA during insertion of the monofilament.
For this reason in this study (n = 30), we inserted 15–18 mm of 4.0 silk suture into the left ICA. We lost only two rats (surgical success rate was 93% (n = 28), and mortality rate was 7%). One animal died due to SAH (complication of monofilament insertion) and the other due to excessive anesthesia. Twenty-eight rats survived at least 4 weeks. We noted that with no coagulation of external carotid branches made rats recover faster. They stood up and reached the ceiling of their cages in the next day.
All of the surgeries in this study were performed by trained neurosurgeons and residents with extensive microsurgical experience. Nevertheless, their relatively limited experience with rat extracranial vascular anatomy and intraluminal placement of the filament resulted initially in the high rate of perioperative mortality. The team also had little experience of anesthesia of small animals and one rat died because excessive anesthesia.
Effect of MCAO
The effects of MCAO were confirmed by the evidence of motor neurological deficit (Bederson test) as shown in
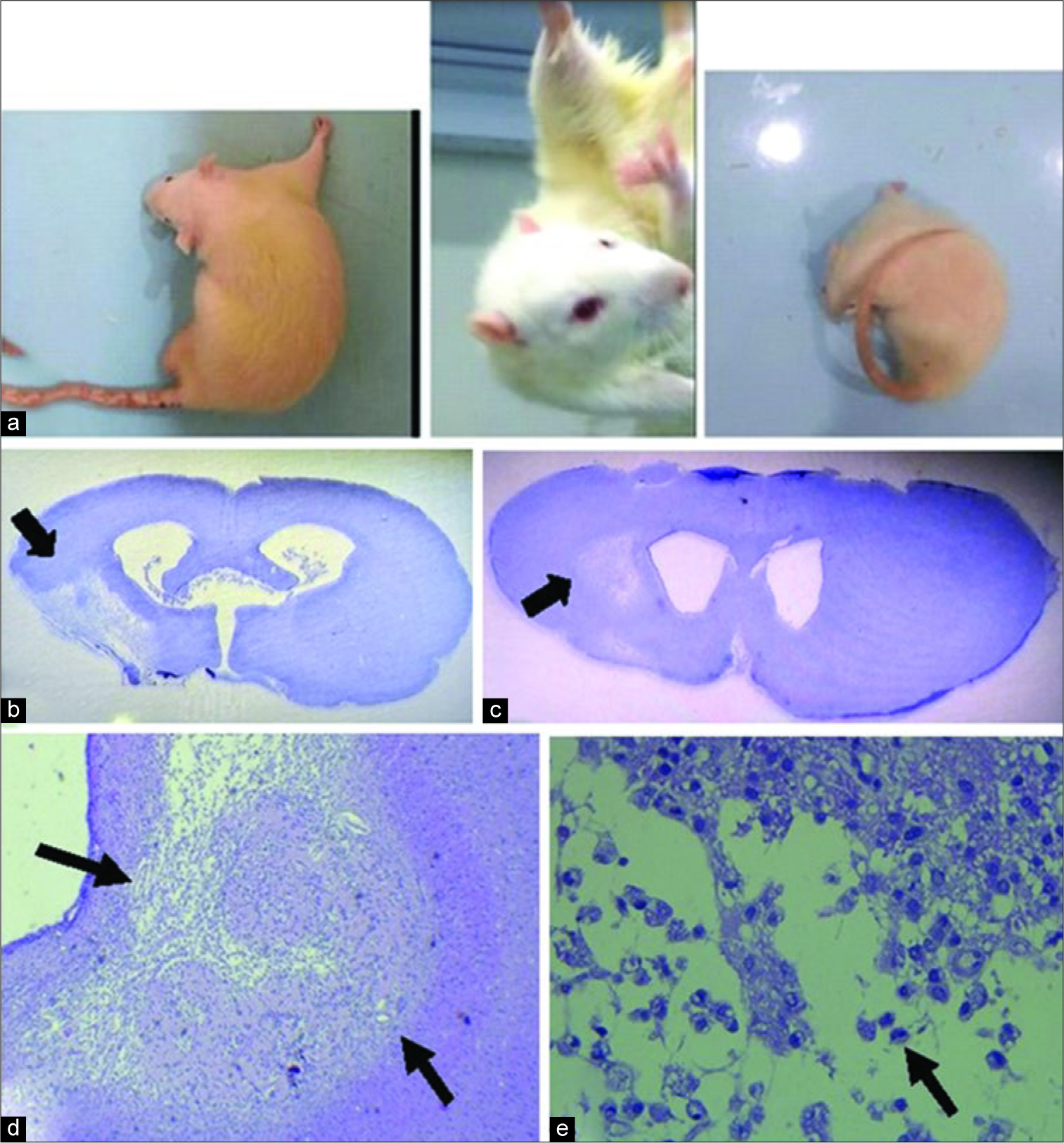
Figure 4:
Behavior test and histopathology findings. (a) Spontaneous circling 1 h after occlusion (b and c) photomicrograph showing a necrotic lesion in the left striatum and cortex after 4 weeks middle cerebral artery occlusion. Black arrows indicate the necrotic lesion H and E. (d) Appearance of cortex necrosis at low magnification, identified by pallor within the zone of black arrows. There is loss of tissue and collapse of the cortical structure after 4 weeks in Wistar rat. (e) The atrophy of the cortex is accompanied by residual macrophages (arrow) and cavitated areas (×40 H and E).
Functional outcome
To evaluate neurological outcomes after induced ischemia, the Bederson assessments were performed before 1.5 h and 24 h after stroke. All assessments were performed independently by other members of the team’s laboratory who had no information about this study.
Rats were evaluated and given deficit scores based on parameters including flexion, lateral push, or circling. The Bederson examination is scored on a scale from 0 to 3.
All the surviving animals showed clear neurological motor deficits within the first 1.5 h after MCAO (100% with score 3) [
Histopathology findings
The histopathological hematoxylin-eosin findings after 4 weeks post-MCAO showed that there was collapse of the cortex [
DISCUSSION
MCAO techniques are acknowledged as standard procedures to produce infarction that resembles the situation in human. Considerable literature describes this technique along with several modifications.[
This study aims to demonstrate that with minimal infrastructure, MCAO procedure could still be performed successfully. Surgical clips designed for rats are not available in Indonesia. The authors tried to undertake the procedure using human aneurismal clips, but the size was not suitable for rat’s neck vessels. Laboratory surgical microscopes are not readily available in Indonesia. The authors used ×8 surgical loupe that is quite affordable compared with a surgical microscope. The microscope used to take photos in this study was a microscope in Neurosurgery Department of Cipto Mangunkusumo Hospital, Jakarta.
As a substitute for surgical clips, the authors used a double knot of 4.0 silk suture. The authors did not find any difficulties in constructing the double knot with the silk suture and there was the added advantage that this suture was not as easily loosened as a human aneurysmal clip. The authors implemented the double knot silk suture in three places (1) ECA, (2) CCA, and (3) ICA. This technique resulted in less bleeding and shortened duration of operation.
Another modification in this study is that there is no coagulation to the branches of ECAs which are OA and superior thyroidea artery. Many previous studies in literature suggest that coagulation of the OA should be undertaken to mobilize the ECA (when the silk suture inserted from the ECA). However, the authors did not experience any difficulties in making the loop around ECA without any branches coagulation.
The ligation of PPA is an important step that was included in this study. The authors found that it guaranteed the insertion of monofilament fiber directly into MCA. Sometimes performing the ligation in PPA was time consuming and tricky. Several authors in this field of literature also note the importance of making a ligation in PPA.[
Ischemic stroke is a very heterogeneous disorder. Mimicking all aspects of human stroke in one animal model is not possible. Although ischemic stroke was shown clinically and histologically in this study, the volume of the infarcted tissue was not measured. If the volume of the infarcted area could be measured on histologic sections, the results might be more reliable. However, the main aim of this study was to describe MCAO techniques which could be used in Indonesia with minimal resources. Volume measurement of infarction was not planned.
We have described a simple successful technique for MCAO with no coagulation, no clips, and no surgical microscope. We showed that making double loop in CCA, ECA, and ICA was enough to prevent massive bleeding and shorten the duration of operation. Moreover, because there was little or no damage to tissue in the area where the ECA is branching, rats recovered more quickly and there were decreased mortality rates. Therefore, we recommend that this technique can be performed in developing countries such as Indonesia which may lack high-cost resources and laboratory equipment.
CONCLUSION
The study demonstrated that without high standard equipment and tools, this technique can be performed and will yield similar results to those obtained with desired standard of (expensive) laboratory equipment. What is needed is adequate surgical training to gain a better picture of the anatomy of rat neck vessels and more understanding of rats anesthesia and handling of small animals.
References
1. Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996. 27: 1616-22
2. Chu K, Kim M, Jung KH, Jeon D, Lee ST, Kim J. Human neural stem cell transplantation reduces spontaneous recurrent seizures following pilocarpine-induced status epilepticus in adult rats. Brain Res. 2004. 1023: 213-21
3. Desland FA, Afzal A, Warraich Z, Mocco J. Manual versus automated rodent behavioral assessment: Comparing efficacy and ease of bederson and garcia neurological deficit scores to an open field video-tracking system. J Cent Nerv Syst Dis. 2014. 6: 7-14
4. Gaziano TA. Reducing the growing burden of cardiovascular disease in the developing world. Health Aff (Millwood). 2007. 26: 13-24
5. Gerriets T, Stolz E, Walberer M, Müller C, Rottger C, Kluge A. Complications and pitfalls in rat stroke models for middle cerebral artery occlusion: A comparison between the suture and the macrosphere model using magnetic resonance angiography. Stroke. 2004. 35: 2372-7
6. Güzel A, Rölz R, Nikkhah G, Kahlert UD, Maciaczyk J. A microsurgical procedure for middle cerebral artery occlusion by intraluminal monofilament insertion technique in the rat: A special emphasis on the methodology. Exp Transl Stroke Med. 2014. 6: 6-
7. Kawamura S, Yasui N, Shirasawa M, Fukasawa H. Rat middle cerebral artery occlusion using an intraluminal thread technique. Acta Neurochir (Wien). 1991. 109: 126-32
8. Koizumi J. Experimental studies of ischemic brain edema. 1. A new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke. 1986. 8: 1-8
9. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989. 20: 84-91
10. Peña-Tapia PG, Díaz AH, Torres JL. Permanent endovascular occlusion of the middle cerebral artery in wistar rats: A description of surgical approach through the internal carotid artery. Rev Neurol. 2004. 39: 1011-6