- Department of General Surgery, Khyber Teaching Hospital, Peshawar, Pakistan
- Department of Neurosurgery, Pakistan Navy Ship Shifa Hospital, Karachi, Pakistan
- Department of Orthopedic Surgery, Khyber Teaching Hospital, Peshawar, Pakistan
Correspondence Address:
Syed Sarmad Bukhari
Department of Orthopedic Surgery, Khyber Teaching Hospital, Peshawar, Pakistan
DOI:10.4103/2152-7806.177891
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Bukhari SS, Junaid M, Rashid MU. Thalassemia, extramedullary hematopoiesis, and spinal cord compression: A case report. Surg Neurol Int 02-Mar-2016;7:
How to cite this URL: Bukhari SS, Junaid M, Rashid MU. Thalassemia, extramedullary hematopoiesis, and spinal cord compression: A case report. Surg Neurol Int 02-Mar-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/thalassemia-extramedullary-hematopoiesis-and-spinal-cord-compression-a-case-report/
Abstract
Background:Extramedullary hematopoiesis (EMH) refers to hematopoiesis outside of the medulla of the bone. Chronic anemia states such as thalassemia can cause hematopoietic tissue to expand in certain locations. We report a case of spinal cord compression due to recurrent spinal epidural EMH, which was treated with a combination of surgery and radiotherapy. Pakistan has one of the highest incidence and prevalence of thalassemia in the world. We describe published literature on diagnosis and management of such cases.
Case Description:An 18-year-old male presented with bilateral lower limb paresis. He was a known case of homozygous beta thalassemia major. He had undergone surgery for spinal cord compression due to EMH 4 months prior to presentation. Symptom resolution was followed by deterioration 5 days later. He was operated again at our hospital with complete resection of the mass. He underwent local radiotherapy to prevent recurrence. At 2 years follow-up, he showed complete resolution of symptoms. Follow-up imaging demonstrated no residual mass.
Conclusion:The possibility of EMH should be considered in every patient with ineffective erythropoiesis as a cause of spinal cord compression. Treatment of such cases is usually done with blood transfusions, which can reduce the hematopoietic drive for EMH. Other options include surgery, hydroxyurea, radiotherapy, or a combination of these on a case to case basis.
Keywords: Extramedullary, hematopoiesis, spinal cord compression, thalassemia
INTRODUCTION
Thalassemia is an autosomal recessive disorder which leads to a chronic form of anemia. Pakistan is included in the list of countries with the highest incidence and prevalence of thalassemia in the world, with estimated 5000–9000 children born each year with the condition.[
Thalassemia treatment in Pakistan is almost always periodic whole blood transfusions coupled with iron chelation.[
Involvement of the spinal cord can be due to EMH in the epidural space or paraspinal muscles with extension into the spinal canal. The usual location of compression is the thoracic spine due to a currently unidentified mechanism, but certain theories have been suggested.[
CASE PRESENTATION
First surgery and initial presentation
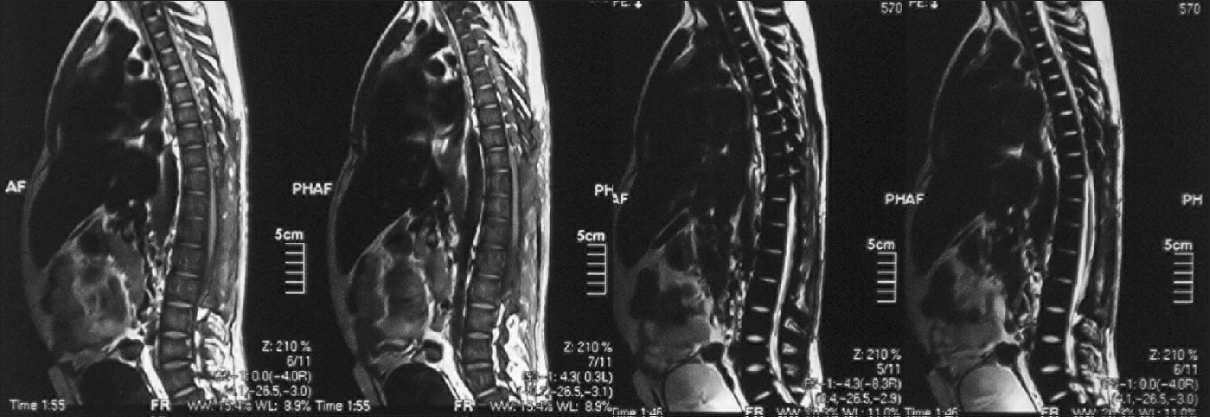
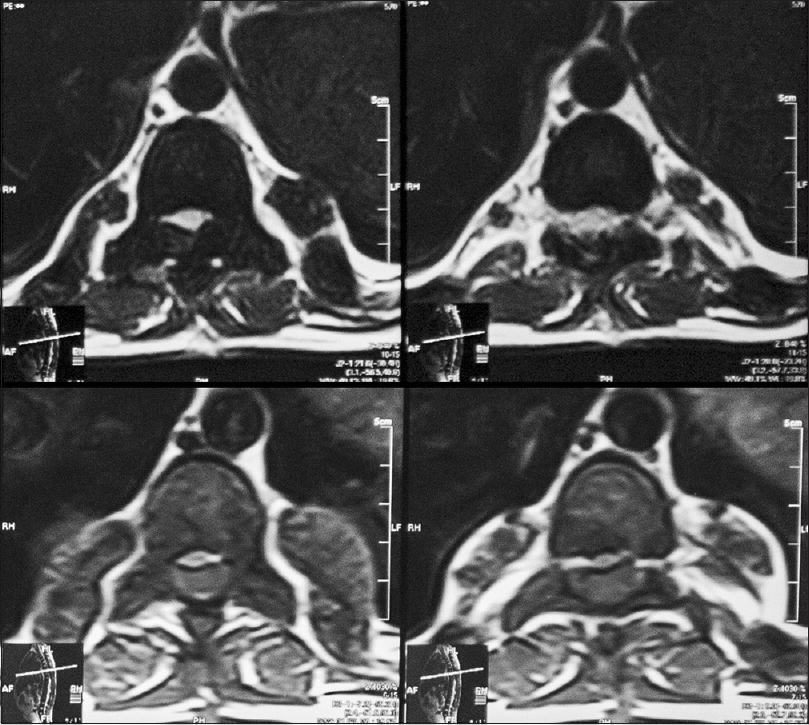
An 18-year-old male patient presented to the neurosurgical clinic with the weakness of both lower limbs and inability to walk for the past 3 months. He had been operated in December of 2011, 4 months prior to presentation at another hospital to relieve paraplegia due to spinal cord compression. He regained full neurological function following the surgery for a period of 5 days after which he progressively declined until he lost function in both legs again. During this period, he underwent regular rehabilitative physiotherapy. Neurological examination showed spastic paraparesis with increased tone in both lower limbs. Power was 0/5. Deep tendon reflexes were 3+ bilaterally in the lower limbs. The sensory level was T12/L1. He was continent for urine and stool and anal tone was normal. A magnetic resonance imaging (MRI) with multi planar imaging of the spine done at presentation [Figures
Review of his available record from the first surgery revealed homozygous beta thalassemia major diagnosed in 1995, treated with regular blood transfusions and iron chelation. Unfortunately, the patient had lost his imaging studies done during his first surgery but reports if the studies were available. His laboratory work showed the following to be outside of normal limits; Hb% 8.5 g%, platelets 130,000/mm3, total bilirubin raised to 2.0 mg/dl (normal value up to 1.0 mg/dl), and alanine aminotransferase raised to 59 IU/L (normal up to 40 IU/L). An ultrasound of the abdomen done, at the time, showed moderately enlarged liver and spleen with no focal lesions, discounting the presence of EMH in these organs. An MRI done prior to the first surgery showed a single extra dural mass extending from T6 to L3, which appeared isointense to hyperintense on T1W and isointense on T2W. The location was posterior and toward the left side with displacement of the spinal cord anteriorly and toward the right. Subtle widening of the left exit canal was noted. Postcontrast images showed the lesion to be heterogeneously enhancing. These scans were unfortunately not available to the patient, but the radiology report was. The patient had undergone open surgery, and three “extradural tumor” specimens had been removed from level T2 to L3. Histopathology revealed normal bone with EMH and no evidence of granulomatous inflammation or malignancy.
Second surgery
The patient was offered repeat surgery since he had shown complete resolution after his first surgery. The patient was placed in prone position with pads and gel foams to achieve a more comfortable position and to prevent pressure necrosis. A dorsal midline skin incision was made exactly over the previous incision. Bilateral laminectomies were done from T4 to T10 with sparing of the facet joints. The affected tissue appeared to be a dark reddish color and moderately adherent to the dura mater of the spinal cord. The masses were peeled away completely from the dura with ease, and no inadvertent durotomy was experienced. Blood loss was about 600 ml with the patient receiving packed cells during surgery. He had an uneventful recovery postoperatively with no complications. He was discharged on postoperative day 5.
Histopathology of the resected tissue revealed bony trabecule with intervening hematopoietic tissue showing hypercellularity with erythroid and myeloid precursors and megakaryocytes. There was no evidence of malignancy.
Postoperative radiotherapy
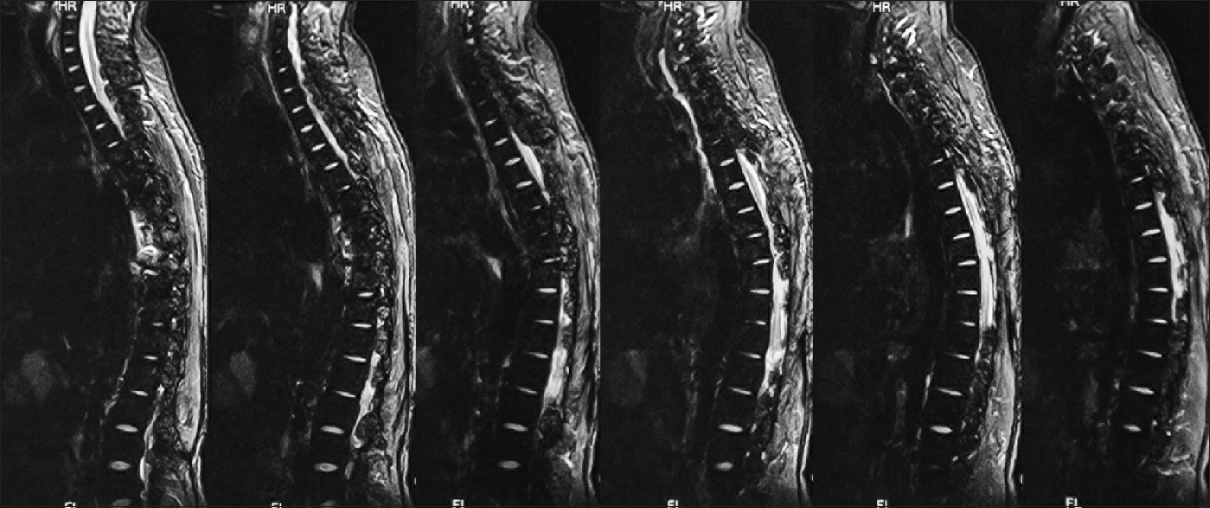
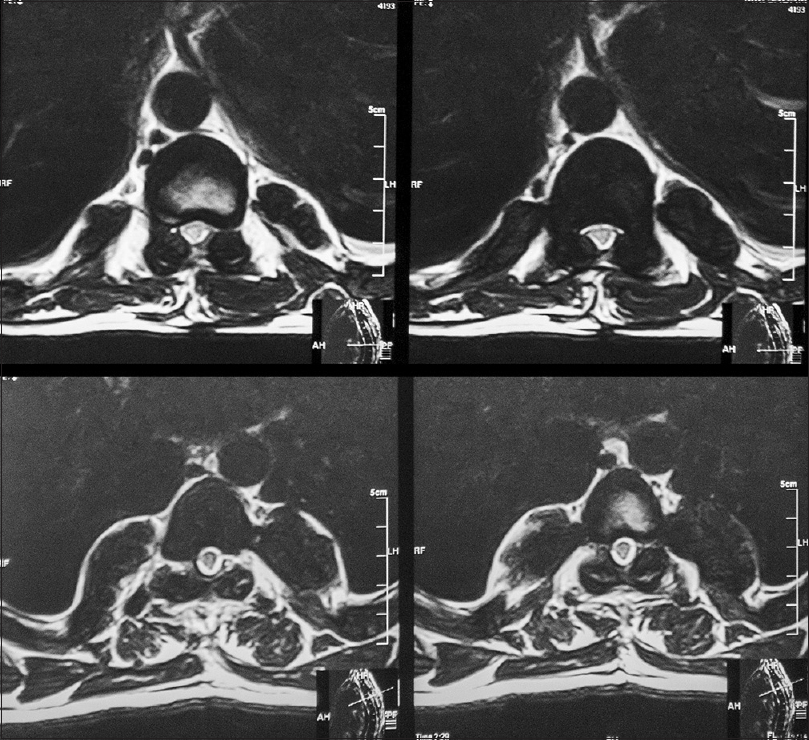
Follow-up at 1 month showed improved strength in both lower limbs (4+/5) with 2+ reflexes, and he was able to walk with support. He was referred to the Institute of Radiology and Nuclear Medicine, Peshawar, where he underwent two cycles of low dose radiotherapy in January 2013 to prevent recurrence. Follow-up at 2 years in October 2014 revealed he had completely recovered strength in both lower limbs and was able to walk without support and was able to function independently. He returned to his job as a computer operator for a private company during this time. MRI done at this time showed no evidence of residual mass or spinal cord compression [Figures
DISCUSSION
Thalassemia is a chronic anemia state that is most often recognized with EMH with the first description of resulting spinal cord compression reported by Gatto et al.[
Formulating the diagnosis
The diagnosis of spinal cord compression due to EMH is usually made by a variety of factors. There is usually a history of chronic hemolytic or anemic disease; however, exceptions have been reported.[
Treatment considerations
Treatment options for EMH include surgery, radiotherapy, blood transfusion, and hydroxyurea. Our management of the patient involved a combination of surgery, radiotherapy, and regular transfusions.[
Patients who have had a more insidious onset of symptoms are preferably treated with more conservative approach with blood transfusions, hydroxyurea, or radiotherapy as a first line treatment, which leads to regression of the EMH. Any one of these is a viable option with comparative studies not yet available.[
Lessons from Pakistan
In a low-income country like Pakistan, blood transfusions seem to be the most cost effective treatment with hydroxyurea obviating the risks associated with blood transfusions. We feel that a collaborative effort is needed in this regard to map out a unified algorithm for the diagnosis, treatment, and rehabilitation of these patients. Moreover, future risk assessment in these patients is warranted.
Recently, a trend toward education and prenatal diagnosis has led to improved knowledge about thalassemia among both the general population and among families with children suffering from the disease.[
CONCLUSIONS
Patients with chronic hematopoietic disorders who suddenly present with spinal cord compression symptoms should be evaluated for EMH since these patients can successfully be treated with blood transfusions without resorting to surgery. Despite a large number of patients at risk for developing this complication every year, there is no algorithm in place in Pakistan to manage them. The current preference is blood transfusions while some surgeons recommend surgical decompression. For the best algorithm, the jury is still out.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ahmed S, Saleem M, Modell B, Petrou M. Screening extended families for genetic hemoglobin disorders in Pakistan. N Engl J Med. 2002. 347: 1162-8
2. Alorainy IA, Al-Asmi AR, del Carpio R. MRI features of epidural extramedullary hematopoiesis. Eur J Radiol. 2000. 35: 8-11
3. Angastiniotis M, Modell B. Global epidemiology of hemoglobin disorders. Ann N Y Acad Sci. 1998. 850: 251-69
4. Ansari SH, Shamsi TS, Ashraf M, Bohray M, Farzana T, Khan MT. Molecular epidemiology of ß-thalassemia in Pakistan: Far reaching implications. Int J Mol Epidemiol Genet. 2011. 2: 403-8
5. Bozdar M, Ahmed S, Jamy OH, Bin Hanif T, Ali N, Khan Khattak SA. Role of genetic counselling in prenatal diagnosis of ß-thalassaemia in Pakistan. J Coll Physicians Surg Pak. 2013. 23: 553-7
6. Cao A, Rosatelli MC, Galanello R. Control of beta-thalassaemia by carrier screening, genetic counselling and prenatal diagnosis: The Sardinian experience. Ciba Found Symp. 1996. 197: 137-51
7. Cario H, Wegener M, Debatin KM, Kohne E. Treatment with hydroxyurea in thalassemia intermedia with paravertebral pseudotumors of extramedullary hematopoiesis. Ann Hematol. 2002. 81: 478-82
8. Cianciulli P, Sorrentino F, Morino L, Massa A, Sergiacomi GL, Donato V. Radiotherapy combined with erythropoietin for the treatment of extramedullary hematopoiesis in an alloimmunized patient with thalassemia intermedia. Ann Hematol. 1996. 72: 379-81
9. Dibbern DA, Loevner LA, Lieberman AP, Salhany KE, Freese A, Marcotte PJ. MR of thoracic cord compression caused by epidural extramedullary hematopoiesis in myelodysplastic syndrome. AJNR Am J Neuroradiol. 1997. 18: 363-6
10. Gatto I, Terrana V, Biondi L. Compression of the spinal cord due to proliferation of bone marrow in epidural space in a splenectomized person with Cooley's disease. Haematologica. 1954. 38: 61-76
11. Hafeez M, Aslam M, Ali A, Rashid Y, Jafri H. Regional and ethnic distribution of beta thalassemia mutations and effect of consanguinity in patients referred for prenatal diagnosis. J Coll Physicians Surg Pak. 2007. 17: 144-7
12. Hashmi MA, Guha S, Sengupta P, Basu D, Baboo S, Neha . Thoracic cord compression by extramedullary hematopoiesis in thalassemia. Asian J Neurosurg. 2014. 9: 102-4
13. Karimi M, Cohan N, Pishdad P. Hydroxyurea as a first-line treatment of extramedullary hematopoiesis in patients with beta thalassemia: Four case reports. Hematology. 2015. 20: 53-7
14. Karimi M, Jamalian N, Yarmohammadi H, Askarnejad A, Afrasiabi A, Hashemi A. Premarital screening for beta-thalassaemia in Southern Iran: Options for improving the programme. J Med Screen. 2007. 14: 62-6
15. Kaufmann T, Coleman M, Giardina P, Nisce LZ. The role of radiation therapy in the management of hematopoietic neurologic complications in thalassemia. Acta Haematol. 1991. 85: 156-9
16. Lau SK, Chan CK, Chow YY. Cord compression due to extramedullary hemopoiesis in a patient with thalassemia. Spine (Phila Pa 1976). 1994. 19: 2467-70
17. Lucarelli G, Galimberti M, Polchi P, Angelucci E, Baronciani D, Giardini C. Marrow transplantation in patients with thalassemia responsive to iron chelation therapy. N Engl J Med. 1993. 329: 840-4
18. Ma SK, Chan JC, Wong KF. Diagnosis of spinal extramedullary hemopoiesis by magnetic resonance imaging. Am J Med. 1993. 95: 111-2
19. Malik M, Pillai LS, Gogia N, Puri T, Mahapatra M, Sharma DN. Paraplegia due to extramedullary hematopoiesis in thalassemia treated successfully with radiation therapy. Haematologica. 2007. 92: e28-30
20. O’Connor JF, Levinthal GN, Sheets R, Mullen KD. Spinal extramedullary hematopoiesis secondary to hepatocellular carcinoma. Case report and literature review. J Clin Gastroenterol. 1997. 25: 466-9
21. Orphanidou-Vlachou E, Tziakouri-Shiakalli C, Georgiades CS. Extramedullary hemopoiesis. Semin Ultrasound CT MR. 2014. 35: 255-62
22. Rachmilewitz EA, Giardina PJ. How I treat thalassemia. Blood. 2011. 118: 3479-88
23. Seddighi AS, Seddighi A. Extramedullary hematopoiesis presenting as a compressive cord and cerebral lesion in a patient without a significant hematologic disorder: A case report. J Med Case Rep. 2010. 4: 319-
24. Smith ZA, Lawton CD, Wong AP, Dahdaleh NS, Nixon AT, Ganju A. Minimally invasive thoracic decompression for multi-level thoracic pathologies. J Clin Neurosci. 2014. 21: 467-72
25. Sohawon D, Lau KK, Lau T, Bowden DK. Extra-medullary haematopoiesis: A pictorial review of its typical and atypical locations. J Med Imaging Radiat Oncol. 2012. 56: 538-44
26. Tan TC, Tsao J, Cheung FC. Extramedullary haemopoiesis in thalassemia intermedia presenting as paraplegia. J Clin Neurosci. 2002. 9: 721-5
27. Thomas ED, Buckner CD, Sanders JE, Papayannopoulou T, Borgna-Pignatti C, De Stefano P. Marrow transplantation for thalassaemia. Lancet. 1982. 2: 227-9
28. Trehan A, Sharma N, Das R, Bansal D, Marwaha RK. Clinicoinvestigational and demographic profile of children with thalassemia major. Indian J Hematol Blood Transfus. 2015. 31: 121-6
29. Zhu G, Wu X, Zhang X, Wu M, Zeng Q, Li X. Clinical and imaging findings in thalassemia patients with extramedullary hematopoiesis. Clin Imaging. 2012. 36: 475-82