- Department of Ear Nose and Throat/Head and Neck Surgery, Maastricht University Medical Center, P. Debyelaan 25, 6229 HX Maastricht, The Netherlands
- Department of Neurosurgery, Maastricht University Medical Center, P. Debyelaan 25, 6229 HX Maastricht, The Netherlands
Correspondence Address:
Gusta van Zwieten
Department of Ear Nose and Throat/Head and Neck Surgery, Maastricht University Medical Center, P. Debyelaan 25, 6229 HX Maastricht, The Netherlands
DOI:10.4103/2152-7806.176134
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Zwieten Gv, Smit JV, Jahanshahi A, Temel Y, Stokroos RJ. Tinnitus: Is there a place for brain stimulation?. Surg Neurol Int 10-Feb-2016;7:
How to cite this URL: Zwieten Gv, Smit JV, Jahanshahi A, Temel Y, Stokroos RJ. Tinnitus: Is there a place for brain stimulation?. Surg Neurol Int 10-Feb-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/tinnitus-is-there-a-place-for-brain-stimulation/
Abstract
Tinnitus is the perception of a “phantom sound” and has a high prevalence. Although many therapies have been investigated within the last decades, there is still no effective standard therapy. Animal studies and human functional imaging studies revealed that tinnitus perception is associated with many complex changes in multiple brain structures. There is growing evidence that brain stimulation might be able to interrupt the local altered neuronal activity and hereby inhibit tinnitus perception. In this editorial review, an update is given on the most promising targets for brain stimulation. Promising structures for stimulation are the dorsal cochlear nucleus, the inferior colliculus and the medial geniculate body of the thalamus. For cortical stimulation, the auditory cortex is considered as a target. Nevertheless, the field is waiting for evidence from well-designed clinical trials, based on supporting evidence from experimental/mechanistic research, to support or discourage the application of brain stimulation in tinnitus.
Keywords: Deep brain stimulation, electric stimulation, neuromodulation, tinnitus, treatment
Currently, up to 15% of the general population suffers chronically from the perception of a “phantom sound”, also known as tinnitus.[
Animal studies and human functional imaging studies revealed that tinnitus perception is associated with many complex changes in several different brain structures. The generally accepted hypothesis is that neuronal changes occur in both auditory and nonauditory brain structures, most often as a compensating mechanism on reduced input from the auditory nerve caused by cochlear hair cell damage, which is associated with hearing loss. These central neuronal changes include an increase in spontaneous firing rate, synchronized activity, bursting activity, and tonotopic reorganization.[
Complex interactions within and between auditory and nonauditory brain structures are present in tinnitus. Every change in neuronal activity causes a cascade of changes in direct and indirect connected brain areas. An important role of the limbic system has been implied, as studies have shown that attention and emotions can influence tinnitus perception.[
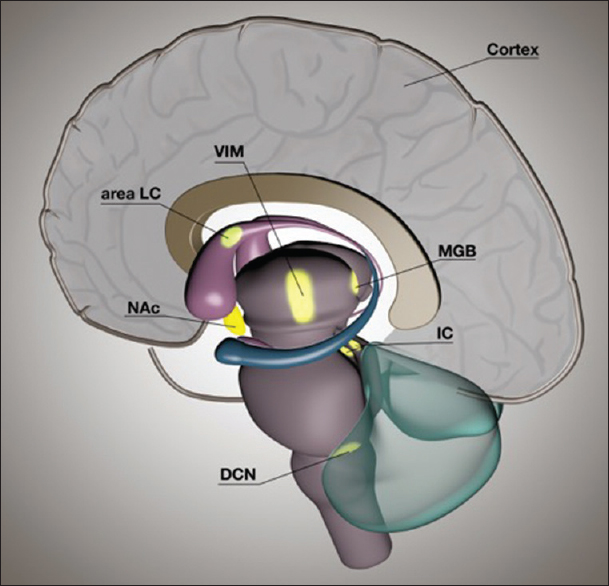
Multiple targets can be proposed in which DBS might have an advantageous effect on tinnitus perception, as shown in
Figure 1
A schematic representation of a sagittal view of a human brain showing possible targets for brain stimulation to treat tinnitus. Auditory structures include the dorsal cochlear nucleus, inferior colliculus, medial geniculate body of the thalamus, and auditory cortex. Nonauditory structures are the nucleus accumbens, locus of caudate neurons (area LC), and ventral intermediate nucleus of the thalamus
Coincidental findings in patients with movement disorders who were treated with DBS, taught us that stimulation of nonauditory targets can attenuate tinnitus. Stimulation of the ventral intermediate nucleus of the thalamus (VIM) in Parkinson's disease patients who also suffered from tinnitus improved tinnitus in three out of seven patients.[
Other neuromodulation-based approaches have also been suggested. In this respect, modulating the activity of relevant cortical structures has been performed. Transcranial magnetic stimulation (TMS) is a noninvasive technique in which strong magnetic field impulses can alter neuronal activity not only in cortical but also in areas connected to the cortex. Repetitive TMS can induce residual inhibition and suppress tinnitus loudness temporarily.[
Besides DBS and cortical neuromodulation approaches, some other concepts have been described. Intracochlear stimulation via cochlear implantation is a viable treatment option in patients with tinnitus and unilateral of bilateral severe or profound hearing loss.[
In conclusion, developments in the field of neuromodulation are promising for patients with severe tinnitus. Several types of neuromodulation-based approaches are being investigated. The general mechanism of action is that neuromodulation interferes with pathological neuronal activity and thereby can attenuate distress or perception of tinnitus. In this respect, increased neuronal activity is found in the DCN, IC, MGB, and auditory cortex. These regions are, therefore, potential targets for brain stimulation. It is impossible to reach these regions selectively and precisely with noninvasive stimulation methods. When surgery is considered, then the MGB is a more accessible target. Furthermore, the MGB is an important relay station where the auditory and limbic structures interact. Tinnitus perception can be influenced by superficial stimulation techniques, which attenuate abnormal auditory cortex activity. Up to date, only a subgroup of tinnitus patients responded to auditory cortex stimulation. From the nonauditory structures, stimulation of the VIM, caudate nucleus (area LC), and NAc have potential to interfere with tinnitus. Using a bottom-up approach with cochlear stimulation or TENS of somatosensory inputs of the DCN, tinnitus percept can be modified in some cases.
Although much is happening at the moment, the field is waiting for evidence from well-designed clinical trials, based on supporting evidence from experimental/mechanistic research, to support or discourage the application of brain stimulation in tinnitus.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors wish to thank Geertjan van Zonneveld for the three-dimensional reconstruction of the potential brain stimulation targets for tinnitus.
References
1. Arts RA, George EL, Griessner A, Zierhofer C, Stokroos RJ. Tinnitus suppression by intracochlear electrical stimulation in single-sided deafness: A prospective clinical trial – Part I. Audiol Neurootol. 2015. 20: 294-313
2. Arts RA, George EL, Stokroos RJ, Vermeire K. Review: Cochlear implants as a treatment of tinnitus in single-sided deafness. Curr Opin Otolaryngol Head Neck Surg. 2012. 20: 398-403
3. Axelsson A, Ringdahl A. Tinnitus – A study of its prevalence and characteristics. Br J Audiol. 1989. 23: 53-62
4. Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res. 2008. 86: 2564-78
5. Benazzouz A, Breit S, Koudsie A, Pollak P, Krack P, Benabid AL. Intraoperative microrecordings of the subthalamic nucleus in Parkinson's disease. Mov Disord. 2002. 17: S145-9
6. Benazzouz A, Hallett M. Mechanism of action of deep brain stimulation. Neurology. 2000. 55: S13-6
7. Brozoski TJ, Ciobanu L, Bauer CA. Central neural activity in rats with tinnitus evaluated with manganese-enhanced magnetic resonance imaging (MEMRI). Hear Res. 2007. 228: 168-79
8. Chen GD, Jastreboff PJ. Salicylate-induced abnormal activity in the inferior colliculus of rats. Hear Res. 1995. 82: 158-78
9. Cheung SW, Larson PS. Tinnitus modulation by deep brain stimulation in locus of caudate neurons (area LC). Neuroscience. 2010. 169: 1768-78
10. Chiken S, Nambu A. Mechanism of deep brain stimulation: Inhibition, excitation, or disruption?. Neuroscientist. 2015. p.
11. de Koning PP, Figee M, van den Munckhof P, Schuurman PR, Denys D. Current status of deep brain stimulation for obsessive-compulsive disorder: A clinical review of different targets. Curr Psychiatry Rep. 2011. 13: 274-82
12. De Ridder D, Vanneste S, Kovacs S, Sunaert S, Menovsky T, van de Heyning P. Transcranial magnetic stimulation and extradural electrodes implanted on secondary auditory cortex for tinnitus suppression. J Neurosurg. 2011. 114: 903-11
13. De Ridder D, Vanneste S. Auditory cortex stimulation might be efficacious in a subgroup of tinnitus patients. Brain Stimul. 2014. 7: 917-8
14. Dehmel S, Cui YL, Shore SE. Cross-modal interactions of auditory and somatic inputs in the brainstem and midbrain and their imbalance in tinnitus and deafness. Am J Audiol. 2008. 17: S193-209
15. Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004. 27: 676-82
16. Engelhardt J, Dauman R, Arné P, Allard M, Dauman N, Branchard O. Effect of chronic cortical stimulation on chronic severe tinnitus: A prospective randomized double-blind cross-over trial and long-term follow up. Brain Stimul. 2014. 7: 694-700
17. Heller AJ. Classification and epidemiology of tinnitus. Otolaryngol Clin North Am. 2003. 36: 239-48
18. Herraiz C, Toledano A, Diges I. Trans-electrical nerve stimulation (TENS) for somatic tinnitus. Prog Brain Res. 2007. 166: 389-94
19. Hoare DJ, Kowalkowski VL, Kang S, Hall DA. Systematic review and meta-analyses of randomized controlled trials examining tinnitus management. Laryngoscope. 2011. 121: 1555-64
20. Kaltenbach JA. Summary of evidence pointing to a role of the dorsal cochlear nucleus in the etiology of tinnitus. Acta Otolaryngol Suppl. 2006. 556: 20-6
21. Kandel ER, Schwartz JH, Jessell TM.editors. Principles of Neural Science. New York: McGraw-Hill; 2000. p.
22. Koller WC, Lyons KE, Wilkinson SB, Troster AI, Pahwa R. Long-term safety and efficacy of unilateral deep brain stimulation of the thalamus in essential tremor. Mov Disord. 2001. 16: 464-8
23. Kraus KS, Canlon B. Neuronal connectivity and interactions between the auditory and limbic systems.Effects of noise and tinnitus. Hear Res. 2012. 288: 34-46
24. Kringelbach ML, Jenkinson N, Owen SL, Aziz TZ. Translational principles of deep brain stimulation. Nat Rev Neurosci. 2007. 8: 623-35
25. Landgrebe M, Azevedo A, Baguley D, Bauer C, Cacace A, Coelho C. Methodological aspects of clinical trials in tinnitus: A proposal for an international standard. J Psychosom Res. 2012. 73: 112-21
26. Langguth B, De Ridder D. Tinnitus: Therapeutic use of superficial brain stimulation. Handb Clin Neurol. 2013. 116: 441-67
27. Langguth B, Landgrebe M, Kleinjung T, Sand GP, Hajak G. Tinnitus and depression. World J Biol Psychiatry. 2011. 12: 489-500
28. Lanting CP, De Kleine E, Bartels H, Van Dijk P. Functional imaging of unilateral tinnitus using fMRI. Acta Otolaryngol. 2008. 128: 415-21
29. Larson PS, Cheung SW. A stroke of silence: Tinnitus suppression following placement of a deep brain stimulation electrode with infarction in area LC. J Neurosurg. 2013. 118: 192-4
30. Leaver AM, Renier L, Chevillet MA, Morgan S, Kim HJ, Rauschecker JP. Dysregulation of limbic and auditory networks in tinnitus. Neuron. 2011. 69: 33-43
31. Lim HH, Lenarz T, Anderson DJ, Lenarz M. The auditory midbrain implant: Effects of electrode location. Hear Res. 2008. 242: 74-85
32. Lowry LD, Eisenman LM, Saunders JC. An absence of tinnitus. Otol Neurotol. 2004. 25: 474-8
33. Manzoor NF, Licari FG, Klapchar M, Elkin RL, Gao Y, Chen G. Noise-induced hyperactivity in the inferior colliculus: Its relationship with hyperactivity in the dorsal cochlear nucleus. J Neurophysiol. 2012. 108: 976-88
34. Matthies C, Thomas S, Moshrefi M, Lesinski-Schiedat A, Frohne C, Battmer RD. Auditory brainstem implants: Current neurosurgical experiences and perspective. J Laryngol Otol Suppl. 2000. 27: 32-6
35. McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism (s) of action of deep brain stimulation: Activation, inhibition, or both. Clin Neurophysiol. 2004. 115: 1239-48
36. Møller A.editors. Epidemiology of tinnitus in adults. Textbook of Tinnitus. New York: Springer-Verlag; 2011. p.
37. Møller AR, Møller MB, Yokota M. Some forms of tinnitus may involve the extralemniscal auditory pathway. Laryngoscope. 1992. 102: 1165-71
38. Ramakers GG, van Zon A, Stegeman I, Grolman W. The effect of cochlear implantation on tinnitus in patients with bilateral hearing loss: A systematic review. Laryngoscope. 2015. 125: 2584-92
39. Rauschecker JP, Leaver AM, Mühlau M. Tuning out the noise: Limbic-auditory interactions in tinnitus. Neuron. 2010. 66: 819-26
40. Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000. 147: 261-74
41. Shi Y, Burchiel KJ, Anderson VC, Martin WH. Deep brain stimulation effects in patients with tinnitus. Otolaryngol Head Neck Surg. 2009. 141: 285-7
42. Shore S, Zhou J, Koehler S. Neural mechanisms underlying somatic tinnitus. Prog Brain Res. 2007. 166: 107-23
43. Shulman A, Strashun AM, Afriyie M, Aronson F, Abel W, Goldstein B. SPECT imaging of brain and tinnitus-neurotologic/neurologic implications. Int Tinnitus J. 1995. 1: 13-29
44. Smit JV, Janssen ML, Schulze H, Jahanshahi A, Van Overbeeke JJ, Temel Y. Deep brain stimulation in tinnitus: Current and future perspectives. Brain Res. 2015. 1608: 51-65
45. Soleymani T, Pieton D, Pezeshkian P, Miller P, Gorgulho AA, Pouratian N. Surgical approaches to tinnitus treatment: A review and novel approaches. Surg Neurol Int. 2011. 2: 154-
46. Song JJ, Vanneste S, Van de Heyning P, De Ridder D. Transcranial direct current stimulation in tinnitus patients: A systemic review and meta-analysis. ScientificWorldJournal 2012. 2012. p.
47. Soussi T, Otto SR. Effects of electrical brainstem stimulation on tinnitus. Acta Otolaryngol. 1994. 114: 135-40
48. Steenerson RL, Cronin GW. Tinnitus reduction using transcutaneous electrical stimulation. Otolaryngol Clin North Am. 2003. 36: 337-44
49. Tyler RS, Rubinstein J, Pan T, Chang SA, Gogel SA, Gehringer A. Electrical stimulation of the cochlea to reduce tinnitus. Semin Hear. 2008. 29: 326-32
50. Vanneste S, De Ridder D. Noninvasive and invasive neuromodulation for the treatment of tinnitus: An overview. Neuromodulation. 2012. 15: 350-60
51. Vanneste S, Plazier M, der Loo EV, de Heyning PV, Congedo M, De Ridder D. The neural correlates of tinnitus-related distress. Neuroimage. 2010. 52: 470-80
52. Vanneste S, Plazier M, Van de Heyning P, De Ridder D. Transcutaneous electrical nerve stimulation (TENS) of upper cervical nerve (C2) for the treatment of somatic tinnitus. Exp Brain Res. 2010. 204: 283-7
53. Vogler DP, Robertson D, Mulders WH. Hyperactivity in the ventral cochlear nucleus after cochlear trauma. J Neurosci. 2011. 31: 6639-45
54. Wu C, Martel DT, Shore SE. Transcutaneous induction of stimulus-timing-dependent plasticity in dorsal cochlear nucleus. Front Syst Neurosci. 2015. 9: 116-






charles tennin
Posted July 9, 2016, 3:06 pm
are their any clinical studies in the los angeles area like at ucla sound frequency neuromodulation for tinnitus ? I have tinnitus it reaches 20,000 hz then does down to 600hz I have had it for 6 weeks now I am almost 73 years of age it came on suddenly an mri showed no growths and the 7th and 8th nerve showed normal can you help me to a program that uses or tests dmb sound frequency theraphy stimulation in the los angeles area I belong to Kaiser permenante, they can do nothing for me to mask the constant noise in my left ear thank you