- Department of Neurosurgery, Institute for Orthopaedics and Neurosciences, Virginia Tech Carilion School of Medicine and Research Institute, Roanoke, Virginia, USA
Correspondence Address:
Michael Benko
Department of Neurosurgery, Institute for Orthopaedics and Neurosciences, Virginia Tech Carilion School of Medicine and Research Institute, Roanoke, Virginia, USA
DOI:10.4103/sni.sni_69_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Eric Marvin, Jordan Synkowski, Michael Benko. Tumor cerebri: Metastatic renal cell carcinoma with dural venous sinus compression leading to intracranial hypertension; a case report. 09-Aug-2017;8:175
How to cite this URL: Eric Marvin, Jordan Synkowski, Michael Benko. Tumor cerebri: Metastatic renal cell carcinoma with dural venous sinus compression leading to intracranial hypertension; a case report. 09-Aug-2017;8:175. Available from: http://surgicalneurologyint.com/surgicalint-articles/tumor-cerebri-metastatic-renal-cell-carcinoma-with-dural-venous-sinus-compression-leading-to-intracranial-hypertension-a-case-report/
Abstract
Background:Pseudotumor cerebri (PTC), also known as idiopathic intracranial hypertension (IIH), is a condition associated with increased intracranial pressure (ICP) in the absence of radiographic findings such as mass lesions or cerebral edema.
Case Description:We describe a case of progressive headache and visual disturbances attributed to PTC that resulted from subacute superior sagittal sinus (SSS) stenosis by a metastatic tumor.
Conclusions:Venous outflow obstruction often presents with an acute symptomatology including infarcts, hemorrhages, and seizures, but only rarely does it cause the progressive development of raised ICP. The sinister presentation of our patient's pathology stemmed from local mass effect caused by a tumor that has hitherto not been reported to cause intracranial hypertension (IH) and was best elucidated using magnetic resonance venography (MRV).
Keywords: Idiopathic intracranial hypertension, papilledema, pseudotumor cerebri, renal cell carcinoma, venous sinus compression
INTRODUCTION
Pseudotumor cerebri (PTC), also known as idiopathic intracranial hypertension (IIH), is a condition associated with increased intracranial pressure (ICP) in the absence of radiographic findings such as mass lesions or cerebral edema.[
CASE DESCRIPTION
VH is a 50-year-old male who presented to an outside facility with complaints of diplopia and headache initially diagnosed as PTC. He was evaluated by ophthalmology and neurology before being transferred to us for further tertiary management of sagittal sinus thrombosis. The patient described mild to moderate headaches of 3 weeks duration with blurry vision and 1 week of double vision that seemed to be exaggerated with rightward gaze. The patient had no pertinent past medical history. His physical exam was remarkable only for bilateral papilledema and subtle right-sided abducens nerve palsy. He was otherwise alert and oriented, with full muscle strength and without myelopathy or other cranial nerve findings. Of note, he had a palpable, compressible soft tissue mass over the vertex of his skull.
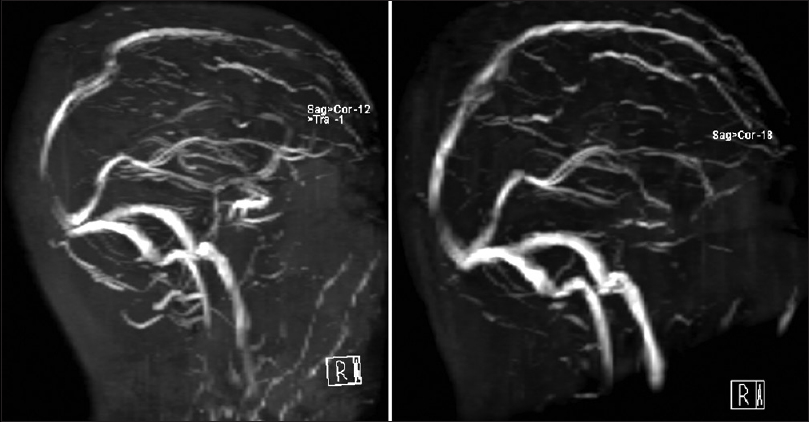
Imaging demonstrated a midline parietal extradural mass with erosion through the skull and into the subgaleal soft tissues. Magnetic resonance imaging (MRI)/MRV demonstrated depression of the SSS with local stenosis in that region. Computed tomography (CT) of the chest, abdomen, and pelvis yielded a 4-cm solid mass on the upper pole of the right kidney. The patient was initially started on a heparin drip by the primary team which was discontinued after definitive imaging was obtained and prior to a diagnostic lumbar puncture (LP). After an opening pressure of 51 cm H2O by manometer in the lateral recumbent position confirmed intracranial hypertension (IH), he was placed on steroids and acetazolamide prior to discussion and recommendation of surgical resection.
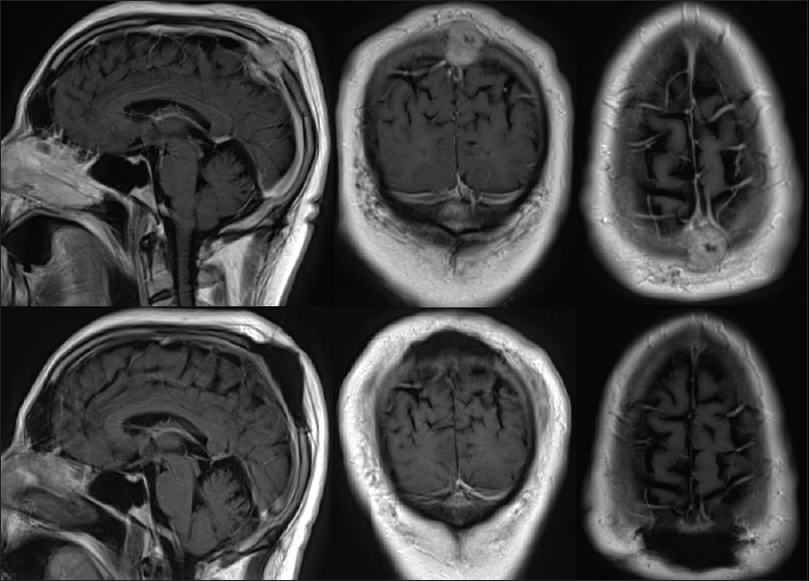
A biparietal craniectomy was performed with gross total resection of the mass as demonstrated in preoperative and postoperative MRI scans [
Figure 1
Preoperative (top) axial, coronal, and sagittal T1 magnetic resonance (MR) with contrast demonstrating an enhancing epidural mass extending through the calvarium and into the subgaleal space with compression of the superior sagittal sinus (SSS). Postoperative (bottom) axial, coronal, and sagittal T1 MR with contrast showing gross total resection of tumor with preservation of the SSS and resolution of sinus stenosis
DISCUSSION
The mechanism of increased ICP in IIH has not been fully elucidated, but the main concepts utilize the Starling resistor hypothesis and the Monro-Kellie doctrine. The latter doctrine explains that because the volume within the cranial compartment is fixed by the rigid confines of the skull, any increase in volume of one of the cranial constituents [brain matter, blood, and cerebrospinal fluid (CSF)] occurs at the expense of the others and the extent of which is at least in part determined by compensatory mechanisms in healthy individuals.[
Papilledema and IH are well known phenomena that can occur with pathology of the dural venous sinuses.[
IH has rarely been described in the context of tumors compressing a dural venous sinus. Case reports include Ewing's sarcoma, plasmacytoma, neuroblastoma, disseminated carcinoma of the breast, and prostate cancer.[
Patients with brain metastases from RCC have a poor prognosis. The average survival time is 3 months if left untreated and 2–9 months if treated with whole brain radiation therapy (WBRT).[
CONCLUSION
In conclusion, when patients present with signs and symptoms resembling PTC, we must reiterate that intracranial mass lesions must first be ruled out as IIH is, by definition, a diagnosis of exclusion.[
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989. 20: 45-52
2. Angeli SI, Sato Y, Gantz BJ. Glomus jugulare tumors masquerading as benign intracranial hypertension. Arch Otolaryngol Head Neck Surg. 1994. 120: 1277-
3. Bennani O, Derrey S, Langlois O, Castel H, Laquerriere A, Freger P. Brain metastasis from renal cell carcinoma. Neurochirurgie. 2014. 60: 12-
4. Brookes G, Graham M. Benign intracranial hypertension complicating glomus jugulare tumor surgery. Am J Otol. 1984. 5: 350-
5. Chopp M, Portnoy H, Branch C. Hydraulic model of the cerebrovascular bed: An aid to understanding the volume-pressure test. Neurosurgery. 1983. 13: 5-11
6. Degnan A, Levy L. Pseudotumor cerebri: Brief review of clinical syndrome and imaging findings. AJNR Am J Neuroradiol. 2011. 32: 1986-
7. Degnan A, Levy L. When Is ‘Idiopathic Intracranial Hypertension’ No Longer Idiopathic?. AJNR Am J Neuroradiol. 2011. 32: 1986-
8. Derosa L, Albiges L, Massard C, Loriot Y, Fizazi K, Escudier B. Safety of available treatment options for renal cell carcinoma. Expert Opin Drug Saf. 2016. 15: 1097-106
9. Farb RI, Vanek I, Scott JN, Mikulis DJ, Willinsky RA, Tomlinson G. Idiopathic intracranial hypertension: The prevalence and morphology of sinovenous stenosis. Neurology. 2003. 60: 1418-
10. Ferrel E, Roehrig A, Kaya E, Carlson J, Ling B, Wagner A. Retrospective study of metastatic melanoma and renal cell carcinoma to the brain with multivariate analysis of prognostic pre-treatment clinical factors. Int J Mol Sci. 2016. 17: 400-
11. Friedman D, Liu G, Digre K. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013. 81:1159: 65-
12. Fuentes S, Metellus P, Levrier O, Adetchessi T, Dufour H, Grisoli F. Depressed skull fracture overlying the superior sagittal sinus causing benign intracranial hypertension. Description of two cases and review of the literature. Br J Neurosurg. 2005. 19: 438-
13. Gironell A, Martí-Fàbregas J, Bello J, Avila A. Non-hodgkin's lymphoma as a new cause of non-thrombotic superior sagittal sinus occlusion. J Neurol Neurosurg Psychiatry. 1997. 63: 121-
14. Holgate R, Hsu C, Scott T. Occlusion of the transverse sinus by meningioma simulating pseudotumor cerebri. Neuro-Ophthalmology. 1987. 7: 113-7
15. Ippen FM, Mahadevan A, Wong ET, Uhlmann EJ, Sengupta S, Kasper EM. Stereotactic radiosurgery for renal cancer brain metastasis: Prognostic factors and the role of whole-brain radiation and surgical resection. J Oncol 2015. 2015. p.
16. Johnston I. The historical development of the pseudotumor concept. Neurosurg Focus. 2001. 11: E2-
17. Johnston I, Kollar C, Dunkley S, Assaad N, Parker G. Cranial venous outflow obstruction in the pseudotumour syndrome: Incidence, nature and relevance. J Clin Neurosci. 2002. 9: 273-
18. Kashimura H, Arai H, Ogasawara K, Ogawa A. Persistent falcine sinus associated with obstruction of the superior sagittal sinus caused by meningioma-case report. Neurol Med Chir (Tokyo). 2007. 47: 83-
19. Kim A, Trobe J. Syndrome simulating pseudotumor cerebri caused by partial transverse venous sinus obstruction in metastatic prostate cancer. Am J Ophthalmol. 2000. 129: 254-
20. Mathews M, Sergott R, Savino P. Pseudotumor cerebri. Curr Opin Ophthalmol. 2003. 14: 364-70
21. Mones R. Increased intracranial pressure due to metastatic disease of venous sinuses. A report of six cases. Neurology. 1995. 15: 1000-
22. Nonne M. Ueber Falle vom Symptomkomplex “Tumor Cerebri” mit Ausgang in Heilung (Pseudotumor Cerebri). Dtsch Z Nervenheil. 1904. 27: 169-216
23. Nunez Bragayrac L, Hoffmeyer J, Abbotoy D, Attwood K, Kauffman E, Spiess P. Minimally invasive cytoreductive nephrectomy: A multi-institutional experience. World J Urol. 2016. 34: 1651-6
24. Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990. 322: 494-
25. Pelton RW, Lee AG, Orengo-Nania SD, Patrinely JR. Bilateral optic disk edema caused by sarcoidosis mimicking pseudotumor cerebri. Am J Ophthalmol. 1999. 127: 229-
26. Peng K, Fuh J, Wang S. High-pressure headaches: Idiopathic intracranial hypertension and its mimics. Nat Rev Neurol. 2012. 8: 700-
27. Plant GT, Donald JJ, Jackowski A, Vinnicombe SJ, Kendall BE. Partial, non-thrombotic, superior sagittal sinus occlusion due to occipital skull tumours. J Neurol Neurosurg Psychiatry. 1991. 54: 520-
28. Platas-Moreno I, Antón-Benito A, Pérez-Cid-Rebolleda MT, Rosado Sierra MB. Papilledema secondary to a superior sagittal sinus thrombosis. Mantle cell lymphoma paraneoplastic syndrome. Arch Soc Esp Oftalmol. 2016. 91: 44-7
29. Powers JM, Schnur JA, Baldree ME. Pseudotumor cerebri due to partial obstruction of the sigmoid sinus by a cholesteatoma. Arch Neurol. 1986. 43: 519-
30. Rendon M. New surgical horizons: The role of cytoreductive nephrectomy for metastatic kidney cancer. Can Urol Assoc J. 2007. 1: S62-
31. Riggeal BD, Bruce BB, Saindane AM, Ridha MA, Kelly LP, Newman NJ. Clinical course of idiopathic intracranial hypertension with transverse sinus stenosis. Neurology. 2013. 80: 289-
32. Shah A, Ivan M, Komotar R. Pseudotumor-like syndrome and cerebrospinal fluid leak in meningiomas involving the posterior third of the superior sagittal sinus: Report of 4 cases. J Neurosurg. 2016. 125: 62-6
33. Ursino M, Lodi C. A simple mathematical model of the interaction between intracranial pressure and cerebral hemodynamics. J Appl Physiol. 1997. 82: 1256-69
34. van den Brink WA, Pieterman H, Avezaat CJ. Sagittal sinus occlusion, caused by an overlying depressed cranial fracture, presenting with late signs and symptoms of intracranial hypertension: Case report. Neurosurgery. 1996. 38: 1044-
35. Wilson M. Monro-kellie 2.0: The dynamic vascular and venous pathophysiological components of intracranial pressure. J Cereb Blood Flow Metab. 2016. 36:1338: 50-
36. Wilson M, Browne JD, Martin T, Geer C. Case report: Atypical presentation of jugular foramen mass. Am J Otolaryngol. 2012. 33: 370-
37. Yri H, Wegener M, Jensen R. Syphilis mimicking idiopathic intracranial hypertension. BMJ Case Rep 2011. 2011. p.