- Department of Neurosurgery, Mainz University Hospital, Langenbeckstraße 1, 55131 Mainz, Germany
Correspondence Address:
Lucas Ezequiel Serrano Sponton
Department of Neurosurgery, Mainz University Hospital, Langenbeckstraße 1, 55131 Mainz, Germany
DOI:10.4103/sni.sni_12_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Lucas Ezequiel Serrano Sponton, Ali Ayyad, Eleftherios Archavlis, Florian Alexander Ringel. Unique case of trigeminal neuralgia due to Epstein-Barr-virus-associated B-cell lymphomatoid granulomatosis of the Meckel's cave and cavernous sinus: Important clinical and therapeutic implications. 26-Jul-2018;9:148
How to cite this URL: Lucas Ezequiel Serrano Sponton, Ali Ayyad, Eleftherios Archavlis, Florian Alexander Ringel. Unique case of trigeminal neuralgia due to Epstein-Barr-virus-associated B-cell lymphomatoid granulomatosis of the Meckel's cave and cavernous sinus: Important clinical and therapeutic implications. 26-Jul-2018;9:148. Available from: http://surgicalneurologyint.com/surgicalint-articles/unique-case-of-trigeminal-neuralgia-due-to-epstein%e2%80%91barr%e2%80%91virus%e2%80%91associated-b%e2%80%91cell-lymphomatoid-granulomatosis-of-the-meckels-cave-and-cavernous-sinus-important/
Abstract
Background:Trigeminal neuralgia (TN) represents one of the most disabling pain syndromes. Several diseases have been described as etiological triggers of TN, vascular compression of the trigeminal nerve being the most frequent cause. Here, we describe for the first time a rare case of TN caused by an infiltration of an isolated Epstein–Barr virus (EBV) B-cell lymphomatoid granulomatosis (LYG) mass into the Meckel's cave and cavernous sinus.
Case Description:A 51-year-old woman undergoing immunosuppressant treatment for Crohn's disease presented due to right-sided TN. Magnetic resonance imaging (MRI) scans revealed an isolated lesion affecting the right Meckel's cave and lateral wall of the cavernous sinus. We accomplished tumor resection through a subtemporal extradural approach and the patient recovered successfully from surgery. Histological examination revealed an LYG, and a blood test confirmed low but positive EBV counts. The immunosuppressant therapy was discontinued and we assumed a watchful waiting management. During a 41-months’ follow-up there was neither evidence of LYG recurrence nor an increase of EBV counts.
Conclusions:LYG, an angiodestructive disease associated with EBV reactivation in the context of immune dysfunction and often associated with an aggressive behavior or even malignant transformation, should be considered as a rare differential diagnosis of TN associated with skull base lesions. The management of this rare disease is still controversial and varies from limiting the treatment to correcting immune dysfunction up to chemotherapy. In this case of an isolated mass, surgical excision and discontinuation of immunosuppressants were effective to prevent the relapse of the disease in a long-term follow-up.
Keywords: B-cell lymphomatoid granulomatosis, cavernous sinus, Meckel's cave, trigeminal neuralgia
INTRODUCTION
Trigeminal neuralgia (TN) represents one of the most disabling pain syndromes that patients can experience. The identification of its etiology in individual patients is mandatory to rule out diseases that may primarily require special evaluation and treatment besides the pharmacological therapy directed to relief neuropathic pain. TN is associated in a vast majority of cases with a vascular compression of the trigeminal nerve, followed more infrequently by inflammatory and tumor processes affecting the nerve or its central pathways.[
CASE REPORT
A 51-year-old woman presented to our clinic because she had been suffering for the lasts 3 weeks from TN affecting the right V1-V2 branch. The patient reported a strong progression of pain as well as diplopia. Her neurological examination was inconspicuous apart from the presence of a right abducens nerve paresis and sensory loss corresponding to the V1 and V2 dermatome. According to her clinical records, she suffered from Crohn's disease and had received azathioprine as immunosuppressive therapy.
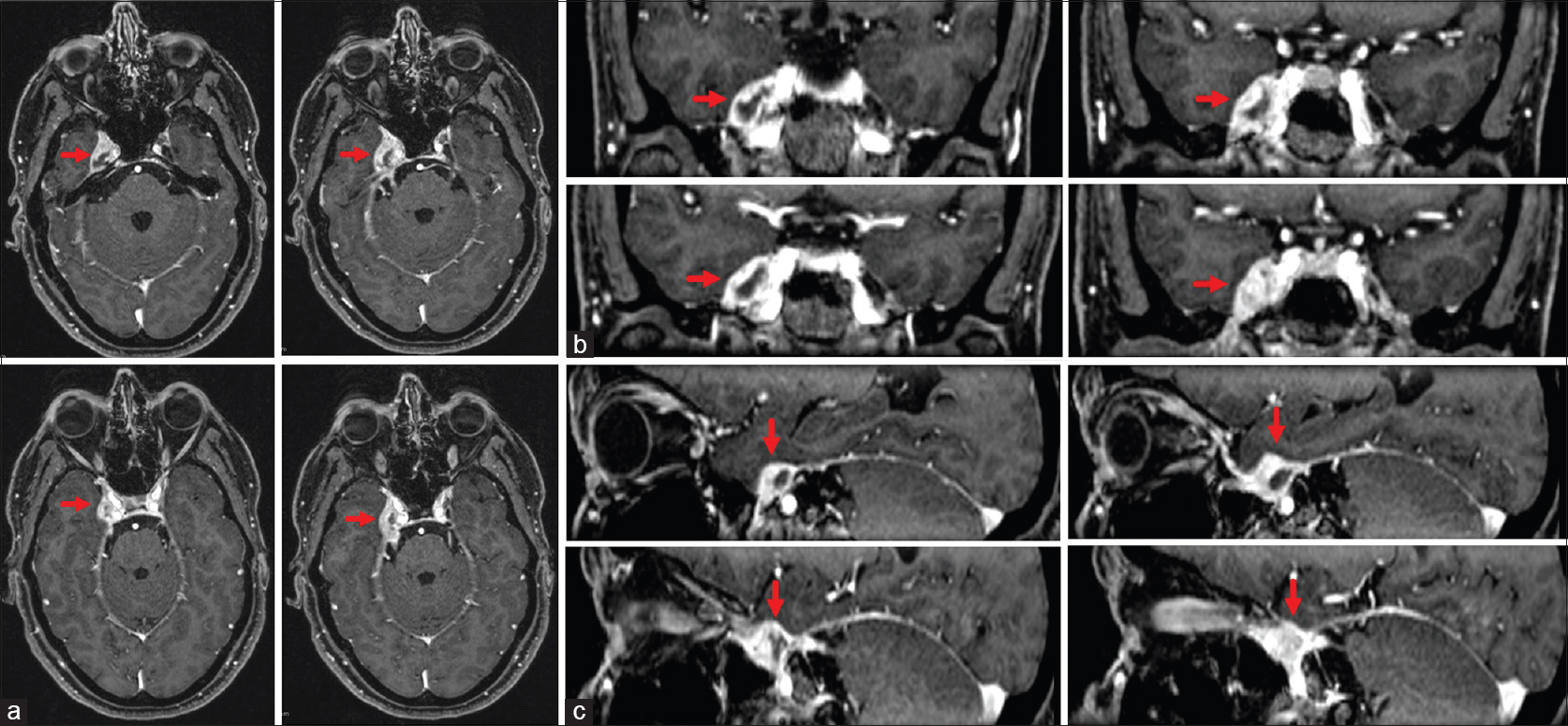
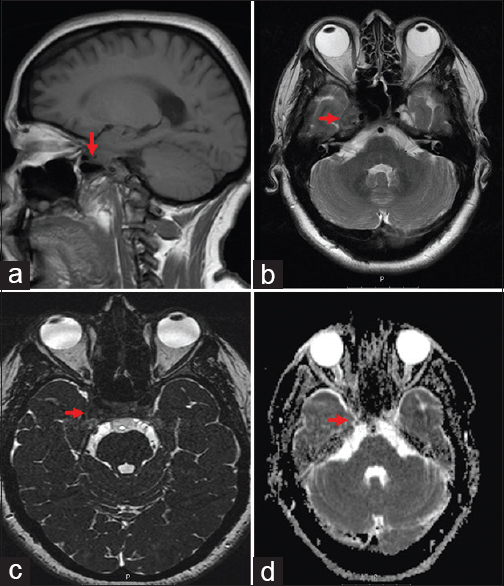
Magnetic resonance imaging (MRI) scans revealed a heterogeneous T1 contrast enhancing lesion in the right Meckel's cave extending to the lateral wall of the cavernous sinus [
Figure 1
Preoperative axial (a), coronal (b), and sagittal (c) MRI scans showing a heterogeneous T1 contrast enhancing lesion involving the right Meckel's cave and the lateral wall of the cavernous sinus corresponding in the histopathological analysis to an Epstein-Barr-virus-associated B-cell lymphomatoid granulomatosis
We suggested surgical exploration and trigeminal nerve decompression in the Meckel's cave using a right subtemporal extradural Dolenc approach. During surgery, the lesion was amenable to be separated from the dural envelope of the cavernous sinus and was completely resected [
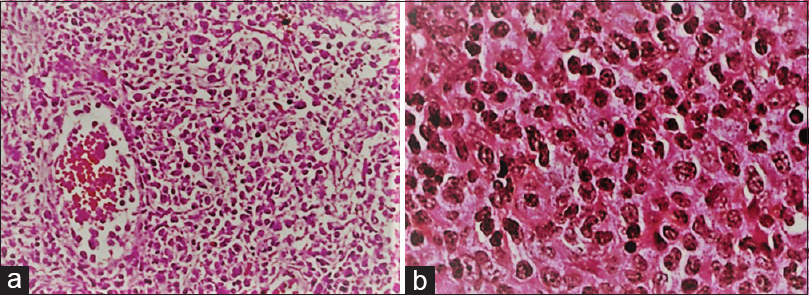
Histopathological examination established the diagnosis of Epstein–Barr-virus-associated B-cell lymphomatoid granulomatosis (LYG) by showing the typical transmural vascular infiltration of lymphocytes, epithelioid cells, and histiocytes with oval nucleus and discrete nucleoles, necrotic epithelioid, and giant cells on the borders and reticulin fiber infiltration [
As expected, analysis of Epstein–Barr virus (EBV) revealed a viral load of 380 copies/mm3 in blood samples. Abdominal and thoracic computed tomography (CT) scans revealed very small intrapulmonary lesions without affecting the lymph nodes. The patient did not present any systemic or respiratory symptoms. A multidisciplinary conference with the colleagues of the hematology and gastroenterology department decided to discontinue azathioprine and start a treatment with rituximab only if the viral load exceeded 103/mm3. The patient was followed up every 3 months in the first year, every 6 months in the second, and then once yearly. In the follow-ups, no corticosteroids were prescribed.
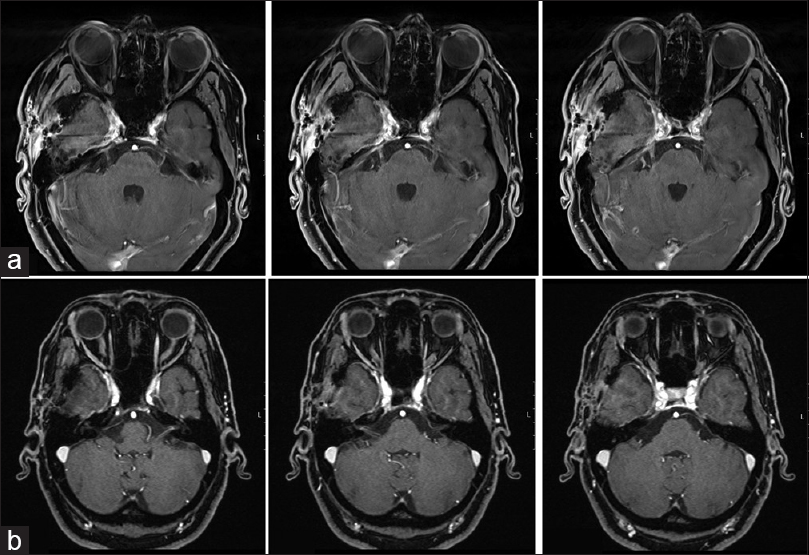
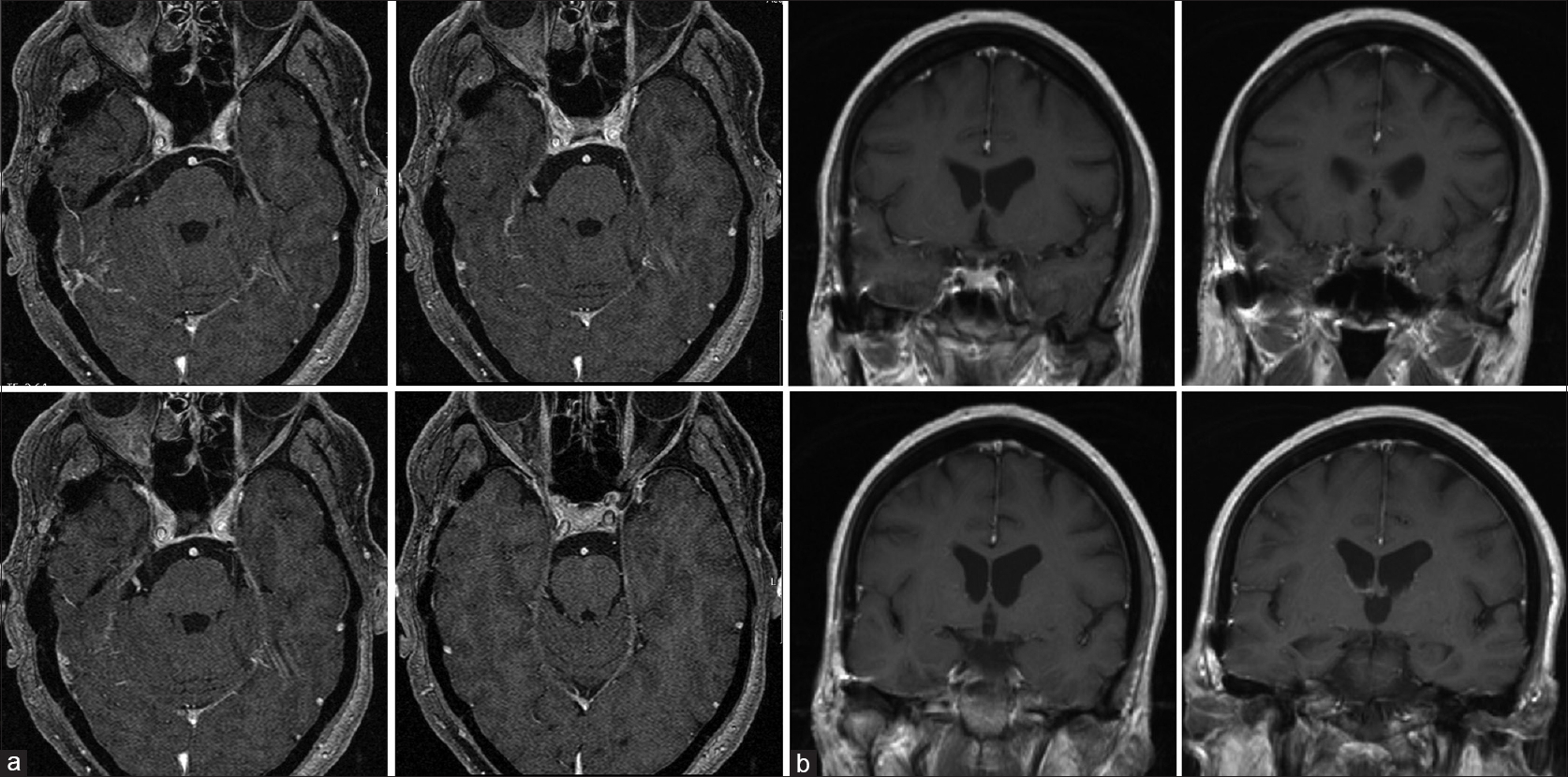
Under surveillance after resection, there was no cranial granuloma recurrence in further neuroimaging studies performed every 6 months during the follow-up [Figures
DISCUSSION
TN is known as one of the most disabling pain syndromes. Although vascular compression of the trigeminal nerve root at the Obersteiner Redlich zone in the cerebellopontine angle can be identified as the cause of TN in 80–90% of the cases, several other conditions, such as demyelinated plaques, tumor and pseudotumor lesions, aneurysms, and arteriovenous malformations, have been identified as triggers of TN.[
Among tumor processes which can affect the trigeminal nerve and trigger TN, neurinoma and meningioma are the most common, although many other entities, included lymphoma, have been already described.[
LYG constitutes an extremely rare angiocentric and angiodestructive process described for the first time by Liebow and colleagues in the early 1970s. It is characterized by nodular lesions composed of a mixed population of lymphoreticular cells showing vascular infiltration and necrosis.[
More than 90% of patients suffering from LYG show a symptomatic bilateral involvement of the lungs.[
Confusion, ataxia, hemiparesis, and seizures are among the most common manifestations of neurological involvement. Others, including cranial nerve dysfunction and peripheral neuropathy affecting usually the lower extremities, have been reported only sporadically. Bell's palsy, diplopia, transient blindness, proptosis, deafness, and vertigo are already described manifestations of cranial nerve dysfunction associated with LYG.[
In the histological examination, mononuclear cells comprising T-lymphocytes, plasma cells, and histiocytes are accompanied by only a small number of B-lymphocytes showing EBV positivity. However, the key feature comprehends the angiocentric and angiodestructive nature of the lesions with transmural vascular infiltration of T-cells and histiocytes showing various degrees of necrosis.[
The pathophysiology of this disease has been linked to a lack of control of EBV-infected B-cells. In this context, it is agreed that recruitment of intravascular T-cells induced by EBV infection may mediate the vascular damage seen in histological specimens.[
The role of surgery may be debated in cases such as the one presented. First, in our patient, surgery was clearly indicated to obtain material for histopathological diagnosis. The atypical localization of a LYG lesion within the Meckel's cave and cavernous sinus had not been described before, thus making the preoperative tentative diagnosis of this disease unlikely in our case. Furthermore, the MRI characteristics shown by this lesion were unspecific. The inhomogeneous contrast enhancement in T1-weighted images resembled features that are commonly described in other diseases which have been reported to affect the cavernous sinus and surrounding structures, such as lymphoma, tuberculoma, sarcoidosis, or Wegener's granulomatosis.[
In addition, the determination of EBV antibodies or a positive viral load can be helpful to suspect this disease, however, these parameters do not replace the need of histopathological examination. EBV infection infect the majority of world population and remains asymptomatic in most cases.[
As surgery was indicated in our patient, the next dilemma we faced was whether just a biopsy for diagnostic purposes or an attempt to remove the mass, followed in both cases by immunosuppressant discontinuation and/or chemotherapy, would be the most reasonable approach for cases similar to the one we describe. This question may be difficult to answer given the infrequent presentation of LYG and it is consequentially difficult to predict a response by only adopting a conservative strategy, considering the relatively poor knowledge we have about the natural history of the disease as well as the lack of similar cases affecting the skull base. Although there have been sporadic reports of LYG remission after discontinuing immunosuppressants or chemotherapy,[
CONCLUSIONS
The case we present here provides relevant clinical and therapeutic implications. We describe for the first time LYG as a rare but existing differential diagnosis of Meckel's cave and cavernous sinus masses associated with TN. As the perioperative evaluation and treatment may significantly differ between LYG and other tumor and inflammatory entities, we recommend being aware of this disease, especially in patients with a prior history of EBV infection, autoimmune disease, and/or undergoing immunosuppressive therapy. In such patients, the presence of systemic symptoms as well as signs of pulmonary, skin, kidney, eye, or even intracerebral and leptomeningeal involvement may be helpful to suspect the diagnosis, but their absence does not rule out LYG, as has been observed in our patient. If LYG is suspected, searching for EBV titers may also be useful to orientate the diagnosis, along with thoracic and abdominal CT scans to detect whether other organs, especially the lungs, are affected. However, in all cases, a histopathological examination is mandatory to confirm the diagnosis.
In our case, we have observed that the removal of LYG localized in the Meckel's cave and cavernous sinus may be feasible and safe. Performed by experienced hands, surgery may correlate with transient pain relief. In our patient, discontinuing immunosuppressive therapy was followed by the reduction and stabilization of EBV counts and a prevention of a relapse or progression of the disease. Whether corticosteroids, rituximab, interferon-α-2b, or chemotherapy should be considered in case of recurrent disease is still an open question. We cannot provide any recommendation for the use of these agents based on our experience.
Ethical standards and conflict of interest
The manuscript does not contain clinical studies or identity patient data and the authors declare that they have no conflict of interest.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Andrews JT, Kountakis SE. Wegener's granulomatosis of the skull base. Am J Otolaryngol. 1996. 17: 349-52
2. Arias M, Iglesias A, Vila O, Brasa J, Conde C. MR imaging findings of neurosarcoidosis of the gasserian ganglion: An unusual presentation. Eur Radiol. 2002. 12: 2723-5
3. Arimoto H, Shirotani T, Nakau H, Hashizume K, Sakai Y, Matsukuma S. Primary malignant lymphoma of the cavernous sinus-case report. Neurol Med Chir (Tokyo). 2000. 40: 275-9
4. Armitage JO. My treatment approach to patients with diffuse large B-cell lymphoma. Mayo Clin Proc. 2012. 87: 161-71
5. Bangiyev L, Kornacki S, Mikolaenko I. Rare isolated trigeminal nerve sarcoidosis mimicking schwannoma. Clin Imaging. 2015. 39: 133-5
6. Braksick S1, Shah-Haque S, El-Haddad B, Moussa R. Neurosarcoidosis presenting as trigeminal neuralgia: A case report and review of the literature. Sarcoidosis Vasc Diffuse Lung Dis. 2013. 30: 153-6
7. Carpentier AS, Riehm S, Charpiot A, Onea A, Debry C, Schultz P. Wegener's granulomatosis of the temporal bone and skull base that mimicked an inflammatory myofibroblastic tumour: A case report. B-ENT. 2010. 6: 135-8
8. Castrale C, El Haggan W, Chapon F, Reman O, Lobbedez T, Ryckelynck JP. Lymphomatoid granulomatosis treated successfully with rituximab in a renal transplant patient. Journal of Transplantation. 2011. 2011: 1-5
9. Cheng TM, Cascino TL, Onofrio BM. Comprehensive study of diagnosis and treatment of trigeminal neuralgia secondary to tumors. Neurology. 1993. 43: 2298-
10. Colby T. Current histological diagnosis of lymphomatoid granulomatosis, Modern Pathology. 2012. 25: 39-42
11. Condon LM, Cederberg LE, Rabinovitch MD, Liebo RV, Go JC, Delaney AS. Age-specific prevalence of Epstein-Barr virus infection among Minnesota children: effects of race/ethnicity and family environment. Clin Infect Dis. 2014. 59: 501-8
12. Dufour H, Diaz A, Metellus P, Fuentes S, Chinot O, Figarella-Branger D. Burkitt lymphoma of the cavernous sinus. Apropos of a case. Neurochirurgie. 2001. 47: 564-7
13. Dunleavy K, Chattopadhyay P, Kawada J, Calattini S, Gostick E, Price D. Immune characteristics associated with lymphomatoid granulomatosis and outcome following treatment with interferon-alpha. Blood. 2010. 116: 424-
14. Fauci AS, Haynes BF, Costa J, Katz P, Wolff SM. Lymphomatoid granulomatosis. Prospective clinical and therapeutic experience over 10 years. N Engl J Med. 1982. 306: 68-74
15. Figueiredo PC, Brock M, De Oliveira AM, Prill A. Arteriovenous malformation in the cerebellopontine angle presenting as trigeminal neuralgia. Arq Neuropsiquiatr. 1989. 47: 61-
16. Goel A, Nadkarni T, Desai AP. Tuberculoma in the Meckel's cave: A case report. Neurol India. 1999. 47: 238-40
17. Huisman TA, Tschirch F, Schneider JF, Niggli F, Martin-Fiori E, Willi UV. Burkitt's lymphoma with bilateral cavernous sinus and mediastinal involvement in a child. Pediatr Radiol. 2003. 33: 719-21
18. Ildan F, Göçer AI, Baǧdatoǧlu H, Uzuneyüpoǧlu Z, Tuna M, Cetinalp E. Isolated trigeminal neuralgia secondary to distal anterior inferior cerebellar artery aneurysm. Neurosurg Rev. 1996. 19: 43-
19. Jaffe ES, Wilson WH. Lymphomatoid granulomatosis: Pathogenesis pathology and clinical implications. Cancer Surv. 1997. 30: 233-48
20. Jaffre S, Jardin F, Dominique S, Duet E, Hubscher P, Genevois A. Fatal haemoptysis in a case of lymphomatoid granulomatosis treated with rituximab. Eur Respir J. 2006. 27: 644-6
21. Jung KH, Sung HJ, Lee JH, Lee KY, Shin JS, Kim KM. A case of pulmonary lymphomatoid granulomatosis successfully treated by combination chemotherapy with rituximab. Chemotherapy. 2009. 55: 386-90
22. Kalina P, Black K, Woldenberg R. Burkitt's lymphoma of the skull base presenting as cavernous sinus syndrome in early childhood. Pediatr Radiol. 1996. 26: 416-7
23. Katzenstein AL, Carrington C, Liebow A. Lymphomatoid granulomatosis: A clinicopathologic study of 152 cases. Cancer. 1979. p. 360-73
24. Keni SP, Wiley EL, Dutra JC, Mellott AL, Barr WG, Altman KW. Skull base Wegener's granulomatosis resulting in multiple cranial neuropathies. Am J Otolaryngol. 2005. 26: 146-9
25. Ko F, Subramanian PS. Orbital and Cavernous Sinus Lymphoma Masquerading as Post-Herpetic Neuralgia. Neuroophthalmology. 2011. 35: 27-31
26. Koubska E, Weichet J, Malikova H. Central nervous system lymphoma: A morphological MRI study. Neuro Endocrinol Lett. 2016. 37: 318-24
27. Kwon EJ, Katz KA, Draft KS, Seykora JT, Rook AH, Wasik MA. Posttransplantation lymphoproliferative disease with features of lymphomatoid granulomatosis in a lung transplant patient. J Am Acad Dermatol. 2006. 54: 657-63
28. Liebow AA, Carrington CR, Friedman PJ. Lymphomatoid granulomatosis. Hum Pathol. 1972. 3: 457-8
29. Love S, Coakham HB. Trigeminal neuralgia: pathology and pathogenesis. Brain. 2001. 124: 2347-
30. Matthies C, Samii M. Management of 1000 vestibular schwannomas (acoustic neuromas): clinical presentation. Neurosurgery. 1997. 40: 1-10
31. Moertel CL, Carlson-Green B, Watterson J, Simonton SC. Lymphomatoid granulomatosis after childhood acute lymphoblastic leukemia: Report of effective therapy Pediatrics. 2001. 107: 82-
32. Patsalides A, Atac G, Hedge U, Janik J, Grant N, Jaffe E, Dwyer A. Lymphomatoid Granulomatosis: Abnormalities of the Brain at MR Imaging. Radiology. 2005. 237: 265-73
33. Quinones-Hinojosa A, Chang EF, Khan SA, McDermott MW. Isolated trigeminal nerve sarcoid granuloma mimicking trigeminal schwannoma: Case report. Neurosurgery. 2003. 52: 700-5
34. Rasper M, Kesari S. Burkitt lymphoma presenting as a rapidly evolving cavernous sinus syndrome. Arch Neurol. 2008. 65: 1668-
35. Roschewski M, Wilson W. Lymphomatoid granulomatosis. The Cancer Journal. 2012. 18: 469-74
36. Sadruddin S, Medeiros LJ, DeMonte F. Primary T-cell lymphoblastic lymphoma of the cavernous sinus. J Neurosurg Pediatr. 2010. 5: 94-7
37. Sanjeevi A, Krishnan J, Bailey PR, Catlett J. Extranodal marginal zone B-cell lymphoma of malt type involving the cavernous sinus. Leuk Lymphoma. 2001. 42: 1133-7
38. Shapiro RS, Chauvenet A, McGuire W, Pearson A, Craft AW, McGlave P. Treatment of B-cell lymphoproliferative disorders with interferon alfa and intravenous gamma globulin. N Engl J Med. 1988. p. 318-1334
39. Stanfield BA, Luftig MA. Recent advances in understanding Epstein-Barr virus. p.
40. Vaphiades MS, Lee AG. Burkitt lymphoma presenting with gingival pain and a cavernous sinus syndrome in an adult. J Neuroophthalmol. 2005. 25: 249-50
41. Wilson WH, Kingma DW, Raffeld M, Wittes RE, Jaffe ES. Association of lymphomatoid granulomatosis with Epstein-Barr viral infection of B lymphocytes and response to interferon-alpha 2b. Blood. 1996. 87: 4531-7