- Department of Neurosurgery, Ehime University School of Medicine, 454 Shitsukawa, Toon, Ehime 791-0295, Japan
- Division of Diagnostic Pathology, Ehime University Hospital, 454 Shitsukawa, Toon, Ehime 791-0295, Japan
Correspondence Address:
Akihiro Inoue
Department of Neurosurgery, Ehime University School of Medicine, 454 Shitsukawa, Toon, Ehime 791-0295, Japan
DOI:10.4103/sni.sni_306_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Tomoki Shinohara, Akihiro Inoue, Shohei Kohno, Yasuo Ueda, Satoshi Suehiro, Shirabe Matsumoto, Masahiro Nishikawa, Saya Ozaki, Seiji Shigekawa, Hideaki Watanabe, Riko Kitazawa, Takeharu Kunieda. Usefulness of neuroimaging and immunohistochemical study for accurate diagnosis of chordoid glioma of the third ventricle: A case report and review of the literature. 02-Nov-2018;9:226
How to cite this URL: Tomoki Shinohara, Akihiro Inoue, Shohei Kohno, Yasuo Ueda, Satoshi Suehiro, Shirabe Matsumoto, Masahiro Nishikawa, Saya Ozaki, Seiji Shigekawa, Hideaki Watanabe, Riko Kitazawa, Takeharu Kunieda. Usefulness of neuroimaging and immunohistochemical study for accurate diagnosis of chordoid glioma of the third ventricle: A case report and review of the literature. 02-Nov-2018;9:226. Available from: http://surgicalneurologyint.com/surgicalint-articles/9060/
Abstract
Background:Chordoid glioma of the third ventricle is a rare neuroepithelial tumor characterized by a unique histomorphology within the third ventricular region, but with radiological and histopathological features mimicking benign lesions such as meningioma. We report a case of chordoid glioma of the third ventricle and suggest a useful indicator for accurate diagnosis.
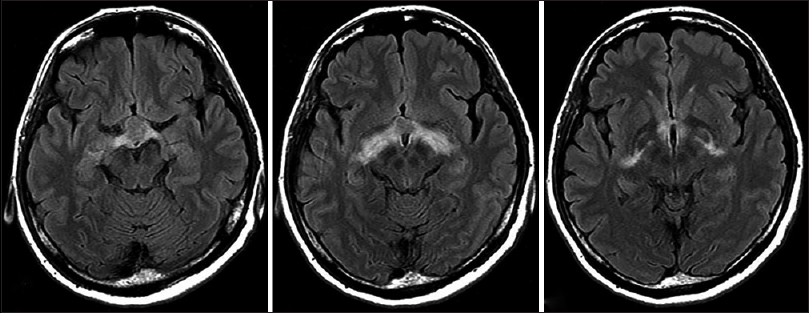
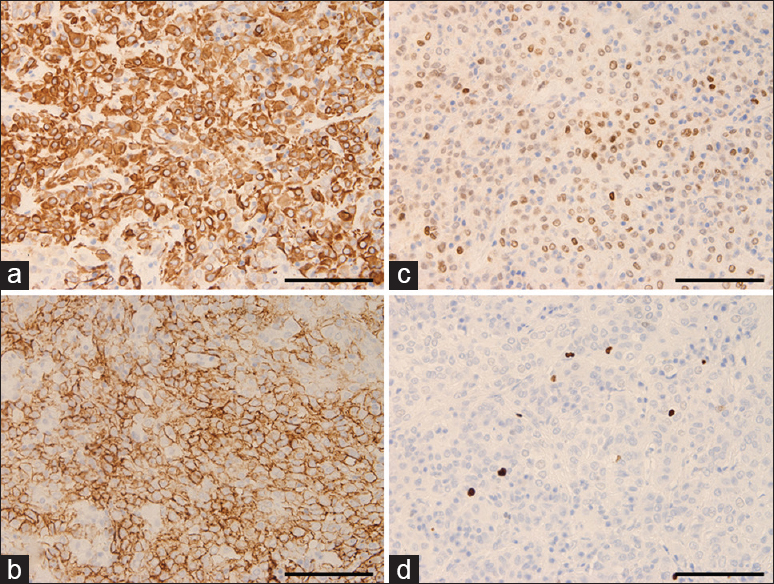
Case Description:A previously healthy 46-year-old woman was admitted to our hospital with mild headache. Neuroimaging revealed a large tumor measuring approximately 18 mm in the suprasellar region, and perifocal edema in the optic tract and internal capsule on magnetic resonance imaging. Laboratory findings revealed no pituitary dysfunction including diabetes insipidus. Gross total resection of the tumor was performed by the interhemispheric translamina terminalis approach. Histological findings revealed nests of regular epithelioid cells with large nuclei and abundant eosinophilic cytoplasm within myxoid stroma. Immunohistochemical studies demonstrated diffuse cytoplasmic expression of glial fibrillary acidic protein (GFAP) and CD34, and strong nuclear staining for thyroid transcription factor 1 (TTF-1). We, therefore, histologically classified the tumor as chordoid glioma of the third ventricle. Headache improved immediately postoperatively, and follow-up neuroimaging after 12 months showed no signs of recurrence.
Conclusions:Chordoid glioma of the third ventricle is a very rare tumor that is difficult to diagnose on routine neuroimaging. Accurate diagnosis requires detailed analysis of neuroimaging and immunohistochemical studies using CD34 and TTF-1 staining.
Keywords: CD34, chordoid glioma of the third ventricle, interhemispheric trans-lamina terminalis approach, perifocal edema in optic tract, thyroid transcription factor 1
INTRODUCTION
Chordoid glioma of the third ventricle is a rare, slow-growing, noninvasive glial tumor in the third ventricle with uncertain histogenesis and chordoid appearance, first described as a clinicopathologic entity by Brat and colleagues in 1998.[
CASE DESCRIPTION
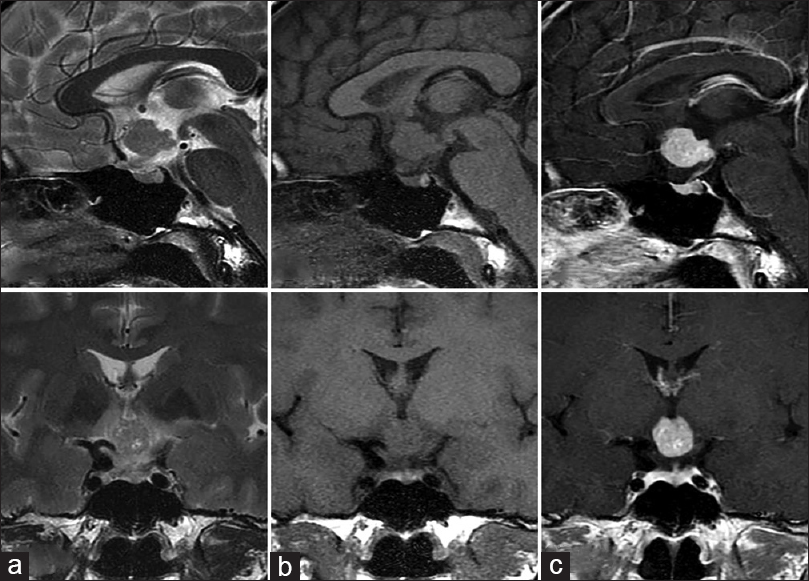
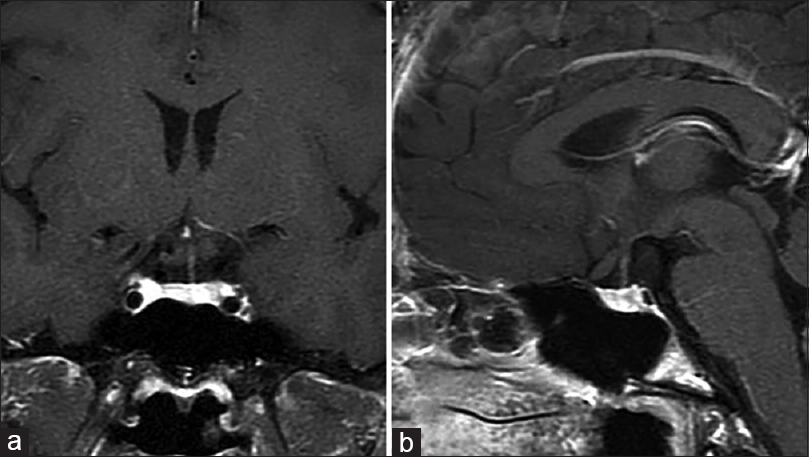
A previously healthy 46-year-old woman presented to our department with a 6-month history of mild headache. Intracranial computed tomography (CT) revealed an iso-dense mass without calcification in the anterior area of the third ventricle. Magnetic resonance imaging (MRI) demonstrated that the tumor (diameter, 14 × 18 × 18 mm) was predominantly isointense on T1-weighted imaging (T1WI) and T2-weighted imaging (T2WI), and homogeneously enhanced to a high degree with gadolinium (Gd) [
Figure 3
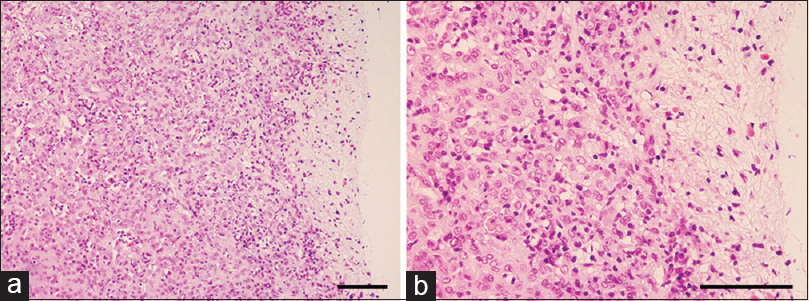
Histopathology of the resected tumor demonstrates solid neoplasms comprising clusters and cords of epithelioid tumor cells within variably mucinous stroma that typically contains a lymphoplasmacytic infiltrate (a and b). Nuclei are round to oval in appearance without evidence of cellular atypia or mitotic figures. Magnification, a) ×200, b) ×400. Scale bar, a) 250 μm, b) 100 μm
DISCUSSION
Chordoid glioma of the third ventricle is a rare tumor with both glial and chordoid features. This tumor is currently recognized as a distinct glioma with grade II malignancy.[
In terms of imaging characteristics, preoperative identification of this tumor with radiological examinations may be difficult. In general, MRI depicts chordoid glioma as isointense on T1WI, and iso- or slightly hyperintense on T2WI. Strong enhancement has been seen after administration of Gd.[
In the 2016 revision of the WHO classification of CNS tumors,[
In terms of treatment, the best treatment strategy for this pathology remains controversial due to its rarity.[
CONCLUSION
We suggest that chordoid glioma of the third ventricle should be included as a differential diagnosis for third ventricular tumors in patients with homogeneously enhancing lesions on MRI. In addition, this entity is difficult to diagnose using routine pathological analysis. Careful identification of signs from preoperative MRI and detailed evaluation of findings from morphological and immunohistochemical studies including CD34 and TTF-1 staining are important for accurate diagnosis and selection of appropriate treatment for chordoid glioma.
Declaration of patient consent
We certify that we have obtained all appropriate patient consent forms. In the form, the patient has consented to her images and other clinical information to be reported in the journal. The patient understands that her names and initials will not be published and due efforts will be made to conceal her identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ampie L, Choy W, Lamano JB, Kesavabhotla K, Mao Q, Parsa AT. Prognostic factors for recurrence and complications in the surgical management of primary chordoid gliomas: A systematic review of literature. Clin Neurol Neurosurg. 2015. 138: 129-36
2. Bongetta D, Risso A, Morbini P, Butti G, Gaetani P. Chordoid glioma: A rare radiologically, histologically, and clinically mystifying lesion. World J Surg Oncol. 2015. 13: 188-
3. Brat DJ, Scheithauer BW, Staugaitis SM, Cortez SC, Brecher K, Burger PC. Third ventricular chordoid glioma: A distinct clinicopathologic entity. J Neuropathol Exp Neurol. 1998. 57: 283-90
4. Desouza RM, Bodi I, Thomas N, Marsh H, Crocker M. Chordoid glioma: Ten years of a low-grade tumor with high morbidity. Skull Base. 2010. 20: 125-38
5. Goode B, Mondal G, Hyun M, Ruiz DG, Lin YH, Van Ziffle J. A recurrent kinase domain mutation in PRKCA defines chordoid glioma of the third ventricle. Nat Commun. 2018. 9: 810-
6. Hewer E, Beck J, Kellner-Weldon F, Vajtai I. Suprasellar chordoid neoplasm with expression of thyroid transcription factor 1: evidence that chordoid glioma of the third ventricle and pituicytoma may form part of a spectrum of lineage-related tumors of the basal forebrain. Hum Pathol. 2015. 46: 1045-9
7. Huo CW, Rathi V, Scarlett A, Galanos J, Wang YY. The trans-laminar terminalis approach reduces mortalities associated with chordoid glioma resections: A case report and a review of 20 years of literature. J Clin Neurosci. 2018. 47: 43-55
8. Jung TY, Jung S. Third ventricular chordoid glioma with unusual aggressive behavior: Case report. Neurol Med Chir (Tokyo). 2006. 46: 605-8
9. Ki SY, Kim SK, Heo TW, Baek BH, Kim HS, Yoon W. Chordoid glioma with intraventricular dissemination: A case report with perfusion MR imaging features. Neuroimaging Head Neck. 2015. 17: 142-6
10. Lee BJ, Cho GJ, Norgren RB, Junier MP, Hill DF, Tapia V. TTF-1, a homeodomain gene required for diencephalic morphogenesis, is postnatally expressed in the neuroendocrine brain in a developmentally regulated and cell-specific fashion. Mol Cell Neurosci. 2001. 17: 107-26
11. Liu WP, Cheng JX, Yi XC, Zhen HN, Fei Z, Li Q. Chordoid glioma: A case report and literature review. Neurologist. 2011. 17: 52-6
12. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007. 114: 97-109
13. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016. 131: 803-20
14. Pasquier B, Peoch M, Morrison AL, Gay E, Pasquier D, Grand S. Chordoid glioma of the third ventricle: A report of two new cases, with further evidence supporting an ependymal differentiation, and review of the literature. Am J Surg Pathol. 2002. 26: 1330-42
15. Raizer JJ, Shetty T, Gutin PH, Obbens E, Holodny AI, Antonescu GR. Chordoid glioma: report of a case with unusual histologic features, ultrastructural study and reviews of the literature. J Neurooncol. 2003. 63: 39-47
16. Rosenberg S, Simeonova I, Bielle F, Verreault M, Bance B, Le Roux I. A recurrent point mutation in PRKCA is a hallmark of chordoid gliomas. Nat Commun. 2018. 9: 2371-
17. Steen R, Egeland T. CD34 molecule epitope distribution on cells of haematopoietic origin. Leuk Lymphoma. 1998. 30: 23-30
18. Tanboon J, Aurboonyawat T, Chawalparit O. A 29-year-old man with progressive short term memory loss. Brain Pathol. 2014. 24: 103-6
19. Wanschitz J, Schmidbauer M, Maier H, Rossler K, Vorkapic P, Budka H. Suprasellar meningioma with expression of glial fibrillary acidic protein. A peculiar variant. Acta Neuropathol. 1995. 90: 539-44
20. Zeinalizadeh M, Sadrehosseini SM, Tayebi Meybodi K, Sharifabadi AH. Expanded endoscopic transnasal approach to the chordoid glioma of the third ventricle: The first case ever reported. J Korean Neurosurg Soc. 2016. 59: 643-6