- Department of Neurosurgery, Wakayama Rosai Hospital, Wakayama, Japan
Correspondence Address:
Nobuhide Hayashi
Department of Neurosurgery, Wakayama Rosai Hospital, Wakayama, Japan
DOI:10.4103/sni.sni_439_17
Copyright: © 2019 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Nobuhide Hayashi, Nagatsuki Tomura, Hideo Okada, Takahiro Sasaki, Eisaku Tsuji, Hiroki Enomoto, Toshikazu Kuwata. Usefulness of preoperative cone beam computed tomography and intraoperative digital subtraction angiography for dural arteriovenous fistula at craniocervical junction: Technical case report. 18-Jan-2019;10:5
How to cite this URL: Nobuhide Hayashi, Nagatsuki Tomura, Hideo Okada, Takahiro Sasaki, Eisaku Tsuji, Hiroki Enomoto, Toshikazu Kuwata. Usefulness of preoperative cone beam computed tomography and intraoperative digital subtraction angiography for dural arteriovenous fistula at craniocervical junction: Technical case report. 18-Jan-2019;10:5. Available from: http://surgicalneurologyint.com/surgicalint-articles/9165/
Abstract
Background:Direct surgery is commonly selected for the treatment of cranio-cervical junction dural arteriovenous fistula and its outcome is more satisfactory than that of embolization. Intraoperative treatment evaluation is relatively easy in embolization, whereas in direct surgery it can be difficult.
Case Description:A 67-year-old male suffered a subarachnoid hemorrhage. On three-dimensional (3D) images of preoperational cone-beam computed tomography (CBCT), the structure of the draining vein was depicted in detail along with the surrounding bone structures. The radial artery penetrated the dura mater, and it was found that there were two veins derived from the radiculospinal vein; one was the anterior radicular vein descending toward the dorsal side (the shallow layer of the surgical field) and the other was the anterior spinal medullary vein ascending toward the ventral side (the deep layer of the surgical field) and flowing out to the anterior spinal vein.
Conclusion:Without detailed assessments with preoperative CBCT, the surgery might have been done with dissection of only the anterior radicular vein in the shallow layers. For identification of the draining vein located deep in the surgical field, such as the cranio-cervical junction, careful assessments using 3D CBCT images are important.
Keywords: Cranio-cervical junction, direct surgery, dural arteriovenous fistula, intraoperative digital subtraction angiography, preoperative cone beam computed tomography
INTRODUCTION
Direct surgery is commonly selected for the treatment of cranio-cervical junction dural arteriovenous fistula (CCJ-dAVF) and its outcome is more satisfactory than that of embolization.[
CASE REPORT
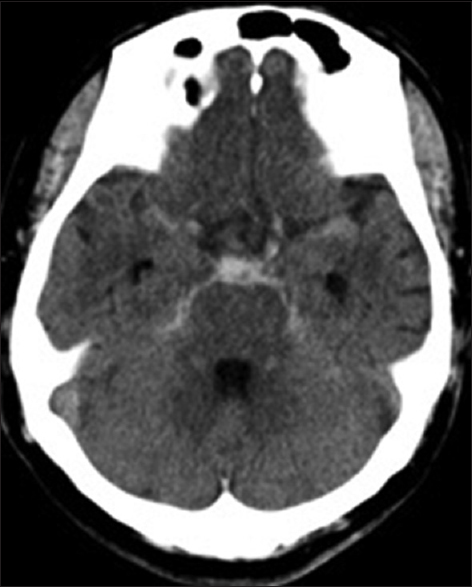
The patient was a 67-year-old male who developed the condition with sudden posterior cervical pain and vomiting before going to bed. As headache and nausea were still present on the following day, he visited the general outpatient clinic by walking. During the physical examination, the patient was clearly conscious without any neurologic deficit symptoms or meningeal irritation symptoms. On head CT, subarachnoid hemorrhage (SAH) was observed [
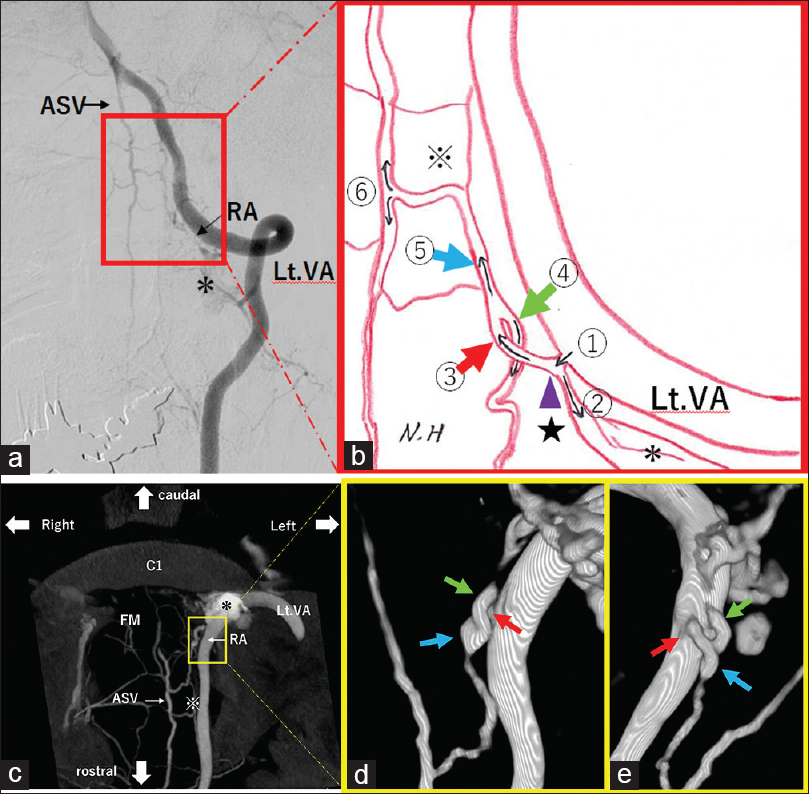
Figure 2
(a): Digital subtraction angiography (DSA) (Anterior-Posterior view) reveals arteriovenous fistula at craniocervical junction. VA: Vertebral Artery, RA: Radicular Artery, ASV: Anterior Spinal Vein (b): Schematic illustration shows detail flows feeding artery to draining vein. (c): Cone beam CT (CBCT) demonstrates the vascular anatomy and clearer definition of the relationships to bone structure. (d and e): Radiculospinal vein is on the back side of anterior radicular vein (d: posterior-anterior view, e: 180 degrees rotated Figure 2d) (*) Epidural Venous Plexus (EVP), ① Radicular artery, ② Radicular artery flows into EVP, (★) Fistulous site (purple arrowhead), ③ Radiculospinal vein (red arrow), ④ Anterior radicular vein (green arrow), ⑤ Anterior spinal medullary vein (blue arrow), (※) Coronal Venous Plexus (CVP), ⑥ Anterior spinal vein, FM: Foramen Magnum, VA: Vertebral Artery, RA: Radicular Artery, ASV: Anterior Spinal Vein
Surgical procedures/intraoperative findings
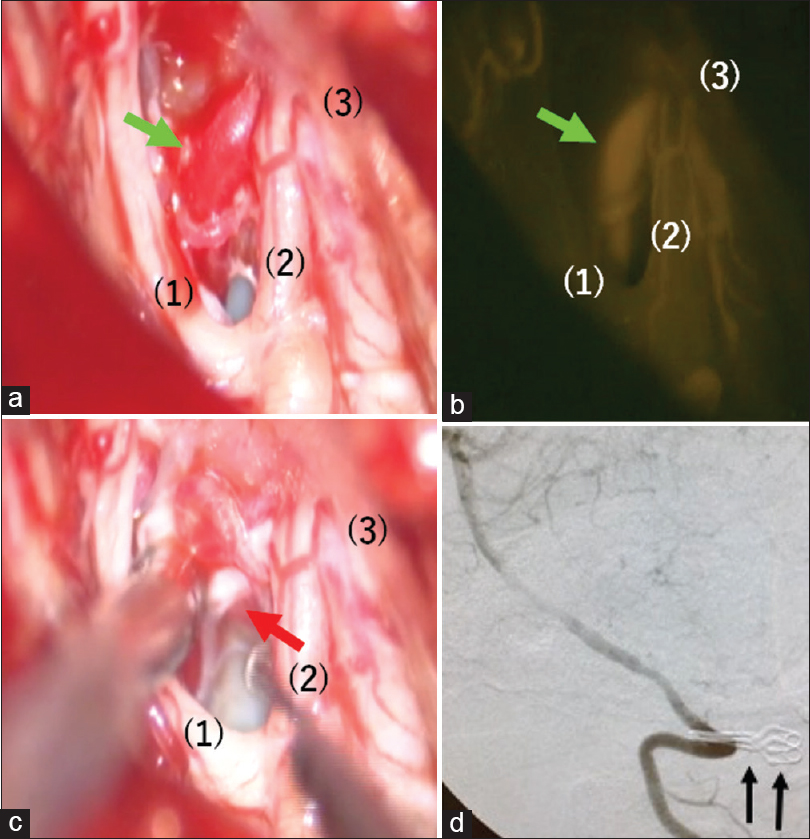
As intraoperative DSA (C-arm) had been scheduled at the same time, a 30 cm-long 4-Fr sheath was placed in the left femoral artery in the supine position under general anesthesia. The head was fixed using MAYFIELD®, which is made of carbon so that it does not affect DSA. Subsequently, lateral suboccipital craniotomy and ipsilateral C1 laminectomy were performed in the prone position. The C1 nerve root was observed near the left vertebral artery, and after dissecting the dura mater, the spinal branch of the accessory nerve and C1 nerve root were observed inside the dura mater. The anterior radicular vein was observed dorsal to the site where the vertebral artery penetrated the dura mater [
Figure 3
Intraoperative view. (a): Anterior radicular vein exhibits red vein (green arrow). (b): Anterior radicular vein exhibits arterial flow by injecting ICG (green arrow). (c): Radiculospinal vein is on the back side of anterior radicular vein and collapsing by suction tube (red arrow). (d): Digital subtraction angiography (DSA) (lateral view) reveals disappearance of venous drainage route and two temporary clips are reflected (black arrows). (1) spinal cord, (2) C1 nerve root, (3) dura matter
Postoperative course
No neurological deficit symptom was observed after the operation. On angiography on day 34 of illness, dAVF had been disappeared, and on day 37 the patient was discharged; he went back home by walking.
DISCUSSION
CCJ-dAVF does not have a venous sinus, which should be embolized, and its feeding artery, which is usually the vertebral artery, is close to the site of the fistula. Therefore, it is not easy to perform intravascular embolization.[
Accurate preoperative imaging evaluation is essential for surgical planning. Vaso CT is very useful for preoperative imaging evaluations, and there are some reports of the use of Dyna CT[
CBCT can even depict AVM nidus behind a hematoma.[
Surgical assistance for vascular lesions include color flow Doppler, ICG, DSA, navigation,[
Doppler is an easy and minimally invasive method which is commonly used for observation of blood flow, but it may not detect the pulse wave accurately due to pulsation, pooling of cerebrospinal fluid/blood, or the angle of the probe. Moreover, it can evaluate only up to cortical arteries; blood flow of small perforating branch cannot be evaluated.[
ICG videoangiography is also an easy and minimally invasive surgical assistance which is useful in both cranial/spinal dAVF.[
Considering the limit of ICG for deep thalamic AVM,[
It is advantageous for intraoperative DSA that it can evaluate not only the surgical field but also extensive cerebral vascular conditions with a large number of people. However, it requires arterial puncture and complex operation of the imaging device. Moreover, it takes time to conduct procedures, resulting in prolongation of the operation time;[
Out of patients who received routine DSA during cerebral aneurysm surgery, only 20% required intraoperative DSA.[
In our patient, a catheter was placed in the left vertebral artery under X-ray immediately before intraoperative DSA. Although it is easier to place the catheter in the supine position right after induction of general anesthesia, we performed catheterization in the prone position immediately before taking the images due to concerns about thromboembolism induced by long-term catheter placement. It might have been easier to perform the left brachial artery puncture rather than the left femoral artery puncture. One study reported that CCJ-dAVF was cured using ICG alone.[
CONCLUSION
We experienced a case which received direct surgery for cranio-cervical junction dural arteriovenous fistula induced by subarachnoid hemorrhage. Deliberate assessment of 3D structures of CBCT was crucial for identification of the draining vein if the surgical field is located deep such as in the cranio-cervical junction.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that his name and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aadland TD, Thielen KR, Kaufmann TJ, Morris JM, Lanzino G, Kallmes DF. 3D C-arm cone beam CT angiography as an adjunct in the precise anatomic characterization of spinal dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2010. 31: 476-80
2. Akdemir H, Öktem S, Menkü A, Tucer B, Tuğcu B, Günaldi O. Image-guided microneurosurgical management of small arteriovenous malformation: Role of neuronavigation and intraoperative doppler sonography. Minim Invasive Neurosurg. 2007. 50: 163-9
3. Amin-Hanjani S, Meglio G, Gatto R, Bauer A, Charbel FT. The utility of intraoperative blood flow measurement during aneurysm surgery using an ultrasonic perivascular flow probe. Neurosurgery. 2006. 58:
4. Bakker NA, Utyttenboogaart M, Luijckx GJ, Eshghi OS, Mazuri A, Metzemaekers JD. Recurrence rates after surgical or endovascular treatment of spinal dural arteriovenous fistulas: A meta-analysis. Neurosurgery. 2015. 77: 137-44
5. Beynon C, Herweh C, Rohde S, Unterberg AW, Sakowitz OW. Intraoperative indocyanine green angiography for microsurgical treatment of a craniocervical dural arteriovenous fistula. Clin Neurol Neurosurg. 2012. 114: 696-8
6. Bilbao CJ, Bhalla T, Dalal S, Patel H, Dehdashti AR. Comparison of indocyanine green fluorescent angiography to digital subtraction angiography in brain arteriovenous malformation surgery. Acta Neurochir (Wien). 2015. 157: 351-9
7. Brailcanu M, Yang W, Caplan JM, Iuang JI. Imaging the spontaneous obliteration of cerebral arteriovenous malformation using c-arm cone beam computed tomography: A case report. Surg Neurol Int. 2015. 6: S399-401
8. Chappell ET, Moure FC, Good MC. Comparison of computed tomographic angiography with digital subtraction angiography in the diagnosis of cerebral aneurysms: A meta-analysis. Neurosurgery. 2003. 52: 624-31
9. Chaudhry NS, Ambekar S, Elhammady MS, Riley JP, Pradilla G, Nogueira RG. Combined use of intraoperative indocyanine green and dynamic angiography in rotational vertebral artery occlusion. J Clin Neurosci. 2016. 30: 152-4
10. Chiang VL, Gailloud P, Murphy KJ, Rigamonti D, Tamargo RJ. Routine intraoperative angiography during aneurysm surgery. J Neurosurg. 2002. 96: 988-92
11. Dashti R, Laakso A, Niemelae M, Porras M, Hernesniemi J. Microscope-integrated near-infrated indocyanine green videoangiography during surgery of intracranial aneurysms: The Helsinki experience. Surg Neurol. 2009. 71: 543-50
12. Dehdashti AR, Thines L, Da Costa LB, terBrugge KG, Willinsky RA, Wallace MC. Intraoperative biplanar rotational angiography during neurovascular surgery. J Neurosurg. 2009. 111: 188-92
13. Derdeyn CP, Moran CJ, Cross DT, Grubb RL Jr, Dacey RG Jr. Intraoperative digital subtraction angiography: A review of 112 consecutive examinations. AJNR Am J Neuroradiol. 1995. 16: 307-18
14. Eesa M, Sharma P, Mitha AP, Sutherland GR, Goyal M. Angiographic computed tomography with selective microcatheterization in delineating surgical anatomy in the case of a dural arteriovenous fistula. J Neurosurg. 2009. 111: 916-8
15. Ferroli P, Acerbi F, Broggi M, Broggi G. Arteriovenous micromalformation of the trigeminal root: Intraoperative diagnosis with indocyanine green videoangiography: Case Report. Neurosurgery. 2010. 67: onsE309-10
16. Fischer G, Stadie A, Oertel JM. Near-infrared indocyanine green videoangiography versus microvascular sonography in aneurysm surgery. Acta Neurochir (Wien). 2010. 152: 1519-25
17. Hänggi D, Etminan N, Steiger HJ. The impact of microscope-integrated intraoperative near-infrared indocyanine green videoangiography on surgery of arteriovenous malformations and dural arteriovenous fistulae. Neurosurgery. 2010. 67: 1094-104
18. Hardesty DA, Thind H, Zabramski JM, Spetzler RF, Nakaji P. Safety, efficacy, and cost of intraoperative indocyanine green angiography compared to intraoperative catheter angiography in cerebral aneurysm surgery. J Clin Neurosci. 2014. 21: 1377-82
19. Hirai T, Korogi Y, Ono K, Murata Y, Suginohara K, Omori T. Preoperative evaluation of intracranial aneurysms: Usefulness of intraarterial 3D CT angiography and conventional angiography with a combined unit—initial experience. Radiology. 2001. 220: 499-505
20. Hiu T, Kitagawa N, Morikawa M, Hayashi K, Horie N, Morofuji Y. Efficacy of Dyna CT digital angiography of the fistulous point of dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2009. 30: 487-91
21. Horie N, Morikawa M, Kitagawa N, Tsutsumi K, Kaminogo M, Nagata I. 2D Thick-section MR digital subtraction angiography for the assessment of dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2006. 27: 264-9
22. Horie N, So G, Debata A, Hayashi K, Morikawa M, Suyama K. Intra-arterial indocyanine green angiography in the management of spinal arteriovenous fistulae. Spine (Philla Pa 1976). 2012. 37: E264-7
23. Irie K, Murayama Y, Saguchi T, Ishibashi T, Ebara M, Takao H. Dynact soft-tissue visualization using an angiographic c-arm system: Initial clinical experience in the operating room. Neurosurgery. 2008. 62: 266-72
24. Killory BD, Nakaji P, Gonzales LF, Ponce FA, Wait SD, Spetzler RF. Prospective evaluation of surgical microscope-integrated intraoperative near-infrated indocyanine green angiography during cerebral arteriovenous malformation surgery. Neurosurgery. 2009. 65: 456-62
25. Klopfenstein JD, Spetzler RF, Kim LJ, Feiz-Erfan I, Han PP, Zabramski JM. Comparison of routine and selective use of intraoperative angiography during aneurysm surgery: A prospective assessment. J Neurosurg. 2004. 100: 230-5
26. Kuroda K, Kinouchi H, Kanemaru K, Wakai N, Senbokuya N, Horikoshi T. Indocyanine green videoangiography to detect aneurysm and related vascular structures buried in subarachnoid clot. J Neurosurg. 2011. 114: 1054-6
27. Kurokawa Y, Ikawa F, Hamasaki O, Hidaka T, Yonezawa U, Komiyama M. A case of cervical spinal dural arteriovenous fistula with extradural drainage presenting with subarachnoid hemorrhage due to a ruptured anterior spinal artery aneurysm. No Shinkei Geka. 2015. 43: 803-11
28. Meckel S, Majer M, Ruiz DS, Yilmaz H, Scheffler K, Radue EW. MR angiography of dural arteriovenous fistulas: Diagnosis and follow up after treatment using a time-resolved 3D contrast-enhanced technique. AJNR Am J Neuroradiol. 2007. 28: 877-84
29. Nishiyama Y, Kinouchi H, Senbokuya N, Kato T, Kanemaru K, Yoshioka H. Endoscopic indocyanine green for video angiography in aneurysm surgery: An innovative method for intraoperative assessment of blood flow in vasculature hidden from microscopic view. J Neurosurg. 2012. 117: 302-8
30. Ogawa A, Furuya K, Ueno T, Naito Y, Nakagomi T. Characteristics and treatment of dural and perimedullary arteriovenous fistula at the craniocervical junction presenting with subarachnoid hemorrhage. No Shinkei Geka. 2012. 40: 121-8
31. Oya S, Nejo T, Fujisawa N, Tsuchiya T, Indo M, Nakamura T. Usefulness of repetitive intraoperative indocyanine green-based videoangiography to confirm complete obliteration of micro-arteriovenous malformations. Surg Neurol Int. 2015. 6: 85-
32. Payner TD, Horner TG, Leipzig TJ, Scott JA, Gilmor RL, Denardo AJ. Role of intra operative angiography in the surgical treatment of cerebral aneurysms. J Neurosurg. 1998. 88: 441-8
33. Raabe A, Nakaji P, Beck J, Kim LJ, Hsu FP, Kamerman JD. Prospective evaluation of surgical microscope-integrated intraoperative near-infrated indocyanine green angiography during aneurysm surgery. J Neurosurg. 2005. 103: 982-9
34. Raabe A, Seidel K. Prevention of ischemic complications during aneurysm surgery. J Neurosurg Sci. 2016. 60: 95-103
35. Rahal JP, Malek AM. Benefit of cone-beam computed tomography angiography in acute management of angiographically undetectable ruptured arteriovenous malformations. J Neurosurg. 2013. 119: 1015-20
36. Schuette AJ, Cawley CM, Barrow DL. Indocyanine green videoangiography in the management of dural arteriovenous fistulae. Neurosurgery. 2010. 67: 658-62
37. Sharma M, Ambekar S, Ahmed O, Nixon M, Sharma A, Nanda A. The utility and limitations of intraoperative near-infrated indocyanine green videoangiography in aneurysm surgery. World Neurosurg. 2014. 82: e607-13
38. Steinmertz MP, Chow MM, Krishnaney AA, Andrews-Hinders D, Benzel EC, Masaryk TJ. Outcome after the treatment of spinal dural arteriovenous fistulae: A contemporary single institution series and meta-analysis. Neurosurgery. 2004. 55: 77-87
39. Stendel R, Pietila T, Al hassan AA, Schilling A, Brock M. Intraoperative microvascular Doppler ultrasonography in cerebral aneurysm surgery. J Neurol Neurosurg Psychiatry. 2000. 68: 29-35
40. Takahashi K, Hayashi S, Ootani T, Sera M, Negishi M. Dural arteriovenous fistula manifesting as subarachnoid hemorrhage at the craniocervical junction. A case report. No Shinkei Geka. 2008. 36: 901-6
41. Takai K, Kin T, Oyama H, Iijima A, Shojima M, Nishido H. The use of 3D computer graphics in the diagnosis and treatment of spinal vascular malformations. J Neurosurg Spine. 2011. 15: 654-9
42. Takami T, Yamagata T, Mitsuhashi Y, Hayasaki K, Ohata K. Direct surgery for spinal arteriovenous fistulas of the filum terminale with intraoperative image guidance. Spine (Phila Pa. 1976. 2012: E1524-8
43. Takeshita T, Hayashi K, Kitagawa N, Suyama K, Nagata I. Dural arteriovenous fistulas at the craniocervical junction fed by bilateral vertebral arteries with intracranial drainage: A case report. No Shinkei Geka. 2009. 37: 1229-33
44. Tang G, Cawley M, Dion JE, Barrow DL. Intraoperative angiography during aneurysm surgery: A prospective evaluation of efficacy. J Neurosurg. 2002. 96: 993-9
45. Tani S, Ikeuchi S, Hata Y, Abe T. Vascular orientation by intra-arterial dye injection during spinal arteriovenous malformation surgery. Neurosurgery. 2001. 48: 240-2
46. Tokunaga S, Sambongi Y, Tsurusaki Y, Tsumoto T. High-resolution cone beam CT localization of an iatrogenic vertebral arteriovenous fistula for trans-arterial target embolization. JNET. 2017. 11: 81-7
47. Wakabayashi K, Yoshizawa M, Kawashima T, Osawa T, Fujimaki H, Asakura K. Dural arteriovenous fistulas at the craniocervical junction fed by multiple arteries: A case report. JNET. 2014. 8: 46-51
48. Wang H, Ye Zp, Huang ZC, Luo L, Chen C, Guo Y. Intraoperative ultrasonography combined with indocyanine green video-angiography in patients with cerebral arteriovenous malformations. J Neuroimaging. 2015. 25: 916-21
49. Wang JY, Molenda J, Bydon A, Colby GP, Coon AL, Tamargo RJ. Natural history and treatment of craniocervical junction dural arteriovenous fistulas. J Clin Neurosci. 2015. 22: 1701-7
50. Yamamoto S, Kim P, Kurokawa R, Itoki K, Kawamoto S. Selective intraarterial injection of ICG for fluorescence angiography as a guide to extirpate perimedullary arteriovenous fistulas. Acta Neurochir (Wien). 2012. 154: 457-63
51. Zaidi HA, Abla AA, Nakaji P, Chowdhry SA, Albuquerque FC, Spetzler RF. Indocyanine green angiography in the surgical management of cerebral arteriovenous malformations: Lessons learned in 130 consecutive cases. Neurosurgery. 2014. 10: 246-51
52. Zhao J, Xu F, Ren J, Manjila S, Bambakidis NC. Dural arteriovenous fistulas at the craniocervical junction: A systemic review. J Neurointervent Surg. 2016. 8: 648-53