- School of Medicine, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, New York, United States.
- Departments of Neurosurgery, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, New York, United States.
- Departments of Radiology, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, New York, United States.
Correspondence Address:
David J. Altschul
Departments of Neurosurgery, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, New York, United States.
DOI:10.25259/SNI_313_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Joseph Dardick, Stephanie Allen, Aleka Scoco, Richard L. Zampolin, David J. Altschul. Virtual reality simulation of neuroendovascular intervention improves procedure speed in a cohort of trainees. 20-Sep-2019;10:184
How to cite this URL: Joseph Dardick, Stephanie Allen, Aleka Scoco, Richard L. Zampolin, David J. Altschul. Virtual reality simulation of neuroendovascular intervention improves procedure speed in a cohort of trainees. 20-Sep-2019;10:184. Available from: http://surgicalneurologyint.com/surgicalint-articles/9659/
Abstract
Background: Realistic virtual reality (VR) simulators have greatly expanded the tools available for training surgeons and interventionalists. While this technology is effective in improving performance in many fields, it has never been evaluated for neuroendovascular procedures. This study aims to determine whether VR is an effective tool for improving neuroendovascular skill among trainees.
Methods: Trainees performed two VR revascularizations of a right-sided middle cerebral artery (MCA) thrombosis and their times to procedural benchmarks (time to enter internal carotid artery [ICA], traverse clot, and complete procedure) were compared. To determine whether the improvement was case specific, trainees with less procedural exposure were timed during VR left-sided ICA (LICA) aneurysm coiling before or after performing MCA thrombectomy simulations. To determine the value of observing simulations, medical students were timed during the right MCA revascularization simulations after watching other VR procedures.
Results: Trainees significantly improved their time to every procedural benchmark during their second MCA revascularization (mean decrease = 1.08, 1.57, and 2.24 min; P = 0.0072, 0.0466, and 0.0230). In addition, time required to access the LICA during aneurysm coiling was shortened by 0.77 min for each previous VR right MCA revascularization performed (P = 0.0176; r2 = 0.71). Finally, medical students’ MCA revascularization simulation times improved by 0.87 min for each prior simulation viewed (P r2 = 0.96).
Conclusion: Both performance and viewing of simulated procedures produced significant decreases in time to reach neuroendovascular procedural benchmarks. These data show that VR simulation is a valuable tool for improving trainee skill in neuroendovascular procedures.
Keywords: Aneurysm, Endovascular, Neuroendovascular, Simulation, Thrombectomy, Virtual reality
INTRODUCTION
The development of numerous practice models and virtual reality (VR) technologies has provided surgeons and interventionalists with unique opportunities to improve technical skill outside of real-life procedures. In particular, VR modalities that recreate real-world scenarios have proven efficacious for endovascular training in other procedural settings,[
This study aims to quantify that affects by exposing trainees with different levels of experience to a VR system that uses haptics and hands-on equipment. Their performance over multiple simulated thrombectomy was measured for improvement. In addition, trainees coiled a VR aneurysm either with or without performing VR thrombectomies beforehand. The effects of the previous thrombectomy on different procedural milestones were calculated. Finally, medical students with no procedural experience attempted VR thrombectomies after watching previous cases and the effect of exposure alone on procedural milestones was determined.
METHODS
VR system
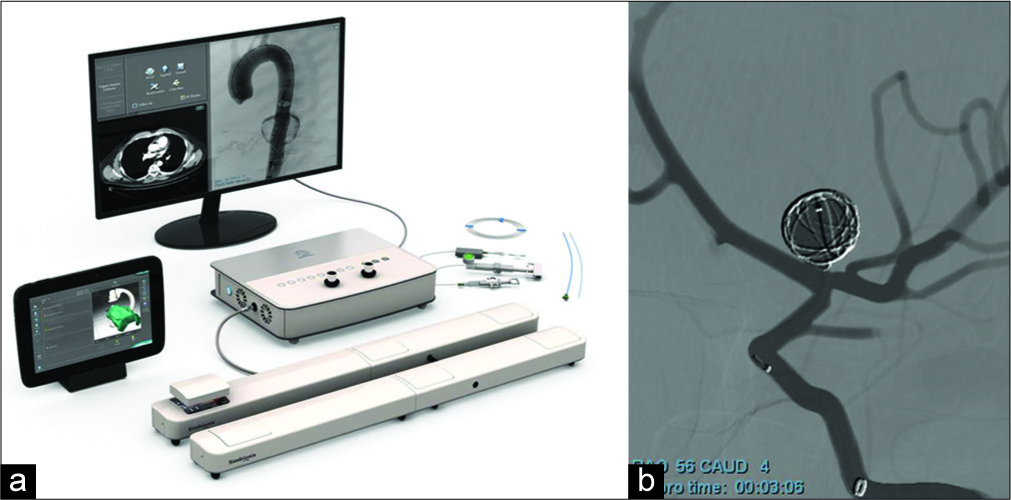
The VR system used was a Simbionix ANGIO Mentor Flex [
VR scenarios and benchmarks
Two virtual scenarios were used in this study. The first was a thrombotic occlusion of the right middle cerebral artery (MCA). For this scenario, times were recorded for when trainees accessed the right internal carotid artery (ICA), traversed the clot, and finished the procedure. The second scenario was an aneurysm coiling of the left- sided ICA (LICA) bifurcation [
Trainee selection
Medical students and neurosurgery and neuroradiology residents at a large academic medical center were informed that there would be two neuroendovascular training seminars over the course of 1 week. During those seminars, trainees were educated on different endovascular devices and invited to participate in simulated cases. Trainees were not told that their times were being recorded to look at improvement for different procedural benchmarks. Participation was voluntary. To be included in the analysis for improvement in revascularization, participants had to perform more than one thrombectomy simulation. Eighteen trainees performed simulated procedures in total. Of those, 7 performed multiple right ICA thrombectomies and 11 performed LICA coiling procedures.
Statistical analysis
All statistical analyses were performed using GraphPad Prism v7. Statistics on procedural benchmarks in thrombectomies was calculated using paired t-tests, but confidence intervals were conservatively calculated using unpaired statistics. Statistics comparing LICA treatment times between groups with less than or greater than 25 previous procedural exposures was done using a Student’s t-test. The relationship between previous thrombectomy simulations and LICA access was calculated using linear regression analysis. The relationship between observed virtual thrombectomies and time to complete the procedure was also calculated using linear regression analysis.
RESULTS
Speed of the right MCA revascularization improves in the second attempt
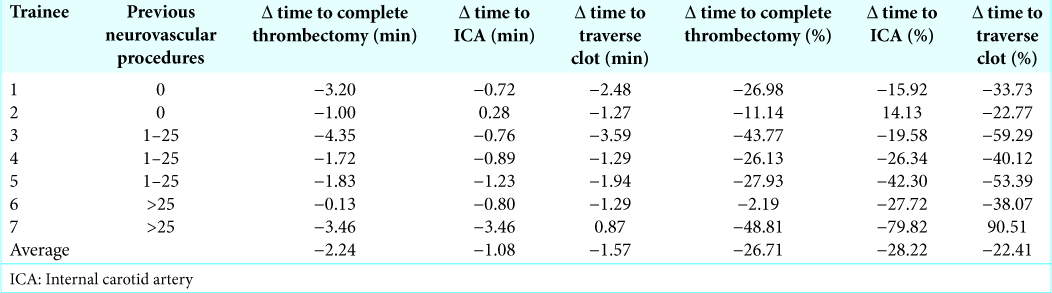
Seven trainees performed two separate thrombectomies over the course of the week. During each procedure, an expert sat next to the trainee and helped guide them. Regardless of the number of previous neuroendovascular procedures that trainees had participated in 0, 1–25, or >25, the time to each procedural landmark decreased during the second simulation [
Performance of the right MCA revascularization improves procedural time point in the left-sided aneurysm coiling
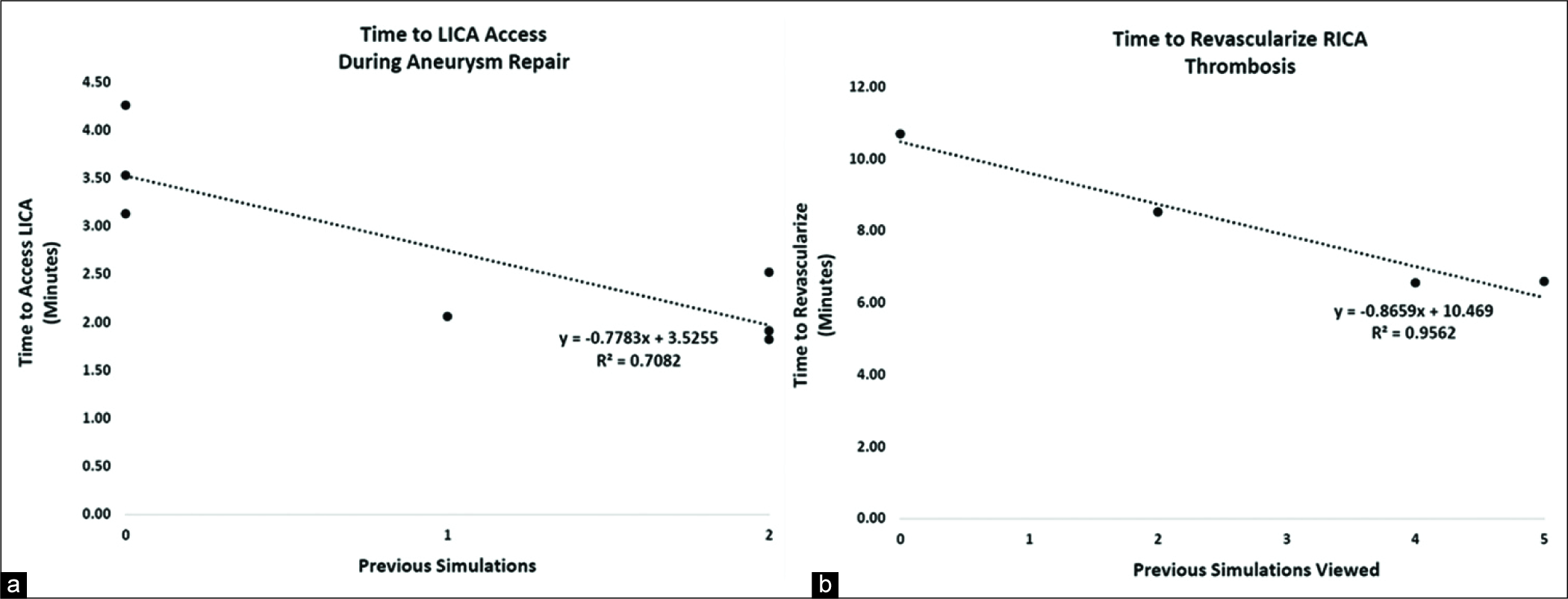
Trainees were invited to perform a LICA coiling regardless of whether they had participated in previous simulation procedures during the seminars. For trainees who had not performed a previous simulated thrombectomy, there was a significant difference (mean<25 = 3.64 min; mean>25 = 1.88 min; P = 0.007; data not shown) between times of trainees who had participated in <25 and those that had participated in >25 procedures. Of trainees who had participated in <25 procedures, three had had not performed a previous VR simulation, one had performed a prior VR MCA revascularization, and three had performed two previous VR MCA revascularizations. In this group, prior VR thrombectomies during the seminars correlated strongly with improved time to accessing the LICA, a technique which required the formation of a Simmons-2 catheter [mean decrease = 0.77 min/prior simulation; P = 0.0176; r2 = 0.71;
Figure 2:
Previous simulated thrombectomy experiences correlate with decreased time to procedural benchmarks in virtual reality (VR) cases. (a) Seven trainees who had participated in fewer than 25 previous neurovascular procedures performed simulated left internal carotid artery (LICA) coiling. For each prior VR thrombectomies done during the seminars, there was a significant decrease in the time required to access the LICA (P = 0.0176, r2 = 0.71). (b) Four medical students with no prior neurovascular training viewed other trainees perform VR thrombectomies. For each prior VR thrombectomy viewed, there was a significant decrease in the time it took students to complete their own VR thrombectomy (P = 0.0221; r2 = 0.96). Statistics was done using linear regression analysis.
Number of observed virtual thrombectomies improves time to complete procedure
Medical students who had never actively participated in previous endovascular procedures were invited to observe other trainees as they performed simulated MCA thrombectomies. When these medical students performed their own MCA thrombectomy simulations, the number of simulations previously viewed correlated significantly with decreased time to treat the clot [mean decrease = 0.87 min/view; P < 0.0221; r2 = 0.96;
DISCUSSION
Stroke is the fifth leading cause of death in the United States and costs over 30 billion dollars annually in health-care expenses and lost productivity.[
One barrier to training that this field shares with other procedural specialties is to provide sufficient procedural experience to practice technical skills. To address this problem, numerous practice models and VR technologies have been developed and used to train physicians.[
CONCLUSION
This study validates that VR neuroendovascular procedures are effective learning tools for trainees at all levels and provides the first quantitative evidence that they improve technical skills. Performing VR thrombectomies with realistic haptics improved trainee speed in homologous procedures and, for trainees with less interventional experience, these effects carried over to the performance of a heterologous procedure – the coiling of a left-sided interior carotid artery aneurysm. In addition, viewing other trainees perform that simulations had a separate positive effect on performance. Together, our results show that VR simulation of endovascular stroke cases is a highly beneficial educational modality that other groups can use to help train incoming professionals. Further studies are needed to see if these results correlate with increased quality of procedure and translate to improvements in real- life procedural outcomes.
References
1. Alaker M, Wynn GR, Arulampalam T. Virtual reality training in laparoscopic surgery: A systematic review and amp; meta-analysis. Int J Surg. 2016. 29: 85-94
2. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015. 372: 11-20
3. Berry M, Reznick R, Lystig T, Lönn L. The use of virtual reality for training in carotid artery stenting: A construct validation study. Acta Radiol. 2008. 49: 801-5
4. . Prevalence of stroke united states, 2006-2010. MMWR Morb Mortal Wkly Rep. 2012. 61: 379-82
5. Crossley R, Liebig T, Holtmannspoetter M, Lindkvist J, Henn P, Lonn L. Validation studies of virtual reality simulation performance metrics for mechanical thrombectomy in ischemic stroke. J Neurointerv Surg. 2019. 11: 775-80
6. Gallagher AG, Ritter EM, Champion H, Higgins G, Fried MP, Moses G. Virtual reality simulation for the operating room: Proficiency-based training as a paradigm shift in surgical skills training. Ann Surg. 2005. 241: 364-72
7. Liebig T, Holtmannspötter M, Crossley R, Lindkvist J, Henn P, Lönn L. Metric-based virtual reality simulation: A paradigm shift in training for mechanical thrombectomy in acute stroke. Stroke. 2018. 49: e239-42
8. Rudarakanchana N, Van Herzeele I, Bicknell CD, Riga CV, Rolls A, Cheshire NJ. Endovascular repair of ruptured abdominal aortic aneurysm: Technical and team training in an immersive virtual reality environment. Cardiovasc Intervent Radiol. 2014. 37: 920-7
9. Rudarakanchana N, Van Herzeele I, Desender L, Cheshire NJ. Virtual reality simulation for the optimization of endovascular procedures: Current perspectives. Vasc Health Risk Manag. 2015. 11: 195-202
10. Saratzis A, Calderbank T, Sidloff D, Bown MJ, Davies RS. Role of simulation in endovascular aneurysm repair (EVAR) training: A Preliminary study. Eur J Vasc Endovasc Surg. 2017. 53: 193-8
11. Wang Y, Guo S, Tamiya T, Hirata H, Ishihara H, Yin X. A virtual-reality simulator and force sensation combined catheter operation training system and its preliminary evaluation. Int J Med Robot. 2017. 13: